1. Background
Polycystic ovary syndrome (PCOS) is a common female disorder affecting women of reproductive age. PCOS is associated with irregular menstrual cycles, weight gain, hirsutism, and infertility. The exact cause of PCOS is not fully understood, but it is believed to involve a combination of genetic, hormonal, and lifestyle factors. Treatment for PCOS typically focuses on managing symptoms and addressing underlying hormonal imbalances (1). A key component of the hypothalamic-pituitary-gonad (HPG) axis, which governs reproductive function, is the control of gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus. Disruption in this regulatory system can lead to hormonal imbalances and irregularities in the menstrual cycle, common features of PCOS (2). Neuropeptides may influence the development and progression of PCOS, and alterations in neuropeptide signaling within the hypothalamus may contribute to the dysregulation of GnRH secretion observed in women with PCOS (3).
Nesfatin-1 is a neuropeptide derived from the precursor protein nucleobindin-2 (NUCB2), consisting of 82 amino acids. It is distributed in the central nervous system, especially in the cerebral cortex, hypothalamus, pancreas, ovary, adipose tissue, and digestive system (4). Research has shown that nesfatin-1 can influence reproductive processes by acting on the HPG axis. Nesfatin-1 has been implicated in the modulation of GnRH/LH secretion, which plays a crucial role in the menstrual cycle and fertility. Evidence indicates that PCOS causes a reduction in the level of nesfatin-1 (5).
Corticotropin-releasing hormone (CRH) is a 41-amino acid peptide. The CRH neurons are located in the hypothalamus, particularly in the paraventricular nucleus (PVN) (6). Research suggests that CRH may influence the pathophysiology of PCOS through its effects on the hypothalamic-pituitary-adrenal (HPA) axis, which controls the production and release of stress-related hormones such as cortisol. Dysregulation of CRH signaling may disrupt the balance of GnRH/LH, ovarian steroidogenesis, ovulation, and fertility in women with PCOS (7).
Naringenin is a flavonoid compound found in certain fruits, particularly citrus fruits such as grapefruits and oranges. This bioflavonoid exists in an inactive form (naringin) in plants, which is converted into its active form (naringenin) by bacteria belonging to the intestinal microbiome. Naringenin belongs to the class of phytochemicals and is structurally similar to 17β-estradiol, which has been associated with various health benefits (8). Its anti-inflammatory, insulin-sensitizing, anti-androgenic, and antioxidant properties make it a promising candidate for managing the symptoms and complications of PCOS (9).
2. Objectives
However, whether and how naringenin exerts its anti-PCOS effects is still unclear. Therefore, the aim of the present research was to investigate the role of naringenin in the regulation of hypothalamic CRH and nesfatin-1 gene expression in a rat model of PCOS.
3. Methods
3.1. Animals
In the present study, adult female Wistar rats (180 - 200 g) were used. The rats were maintained in a laboratory under standard environmental conditions, with a temperature of 22 ± 2°C and a 12-hour dark/light cycle. They had free access to food and water.
3.2. Polycystic Ovary Syndrome Induction
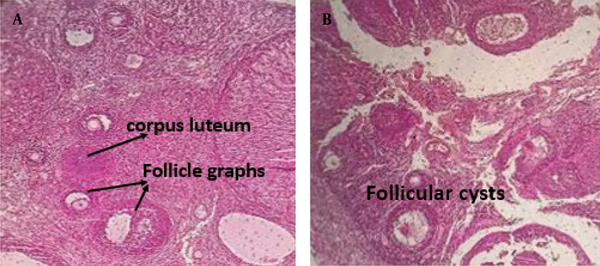
A vaginal smear was performed to monitor the regular estrous cycle (proestrus, estrus, metestrus, and diestrus) for two weeks. To induce PCOS in the estrus phase, 2 mg of estradiol valerate dissolved in 0.2 mL of sesame oil was injected intramuscularly into each rat. Vaginal smears were then taken every 15 days. Finally, PCOS induction was confirmed 60 days after the estradiol valerate injection by examining vaginal cells under a light microscope. The presence of persistent cornified epithelial cells indicated the development of PCOS. Additionally, PCOS was confirmed by hematoxylin-eosin staining of ovarian tissue (Figure 1) (10).
3.3. Experimental Procedure
The rats were classified into four groups (n = 5). The injections were administered as follows: Group I served as the control and received 0.2 mL saline; Group II served as the PCOS group and received 0.2 mL saline; Groups III and IV received naringenin at doses of 20 and 50 mg/kg (IP), respectively, for 14 days.
3.4. Hypothalamic Sample Dissection
First, the animals were euthanized, and the skull was carefully opened to remove the brain. The hypothalamus region was identified according to the Watson-Paxinos atlas. The brain was positioned with the ventral surface facing up, and a 4 mm thick slice containing the hypothalamus was dissected (extending from the front near the optic chiasma, posteriorly to the mammillothalamic region, and laterally to the hypothalamic sulcus). The hypothalamic samples were then removed and immediately stored at -80°C.
3.5. Real-time Polymerase Chain Reaction
Total RNA was extracted from the tissue samples using TRIzol reagent. Reverse transcription from RNA to cDNA was then performed following the instructions provided with the cDNA synthesis kit (Biotech Rabbit, Germany). For RT-PCR, the SYBR Green master mix kit was used (Takara, Japan). The PCR protocol was set as follows: An initial cycle at 95°C for 900 seconds, followed by 40 cycles of denaturation at 95°C for 20 seconds, annealing at 60°C for 15 seconds, and extension at 72°C for 10 seconds. The amplified products for CRH, nesfatin-1, and GAPDH were 103, 204, and 120 base pairs, respectively. Primer sequences are provided in Table 1. The 2−ΔΔCT formula was applied to calculate relative mRNA expression.
| Variables | Primers Sequences |
|---|---|
| nesfatin-1 | |
| Sense | 5′- TGCAGAGAAGAACGCACCAG -3′ |
| Antisense | 5′- ACAGTACCGTGCTTGGATGG -3′ |
| CRH | |
| Sense | 5'- TGGATCTCACCTTCCACCTTCTG -3' |
| Antisense | 5'- CCGATAATCTCCATCAGTTTCCTG -3' |
| GAPDH | |
| Sense | 5′- AAGTTCAACGGCACAGTCAAG -3′ |
| Antisense | 5′- CATACTCAGCACCAGCATCAC -3′. |
Sequence of Sense and Antisense Primers
3.6. Statistical Analysis
SPSS software (version 16) was used to analyze the data. One-way ANOVA and Tukey's post-hoc tests were utilized to assess significant differences between groups. The findings are presented as mean ± SEM, with values considered statistically significant at P ≤ 0.05.
4. Results
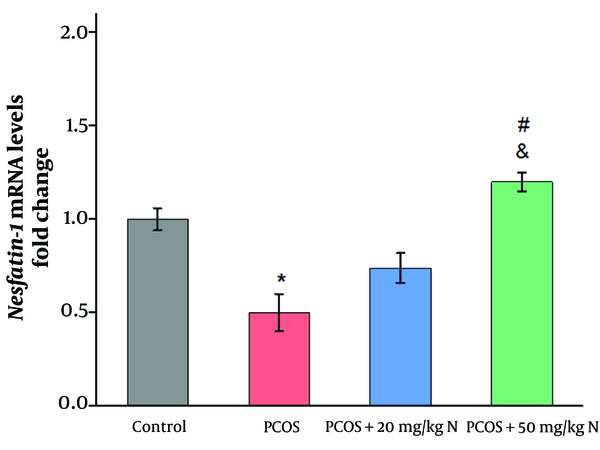
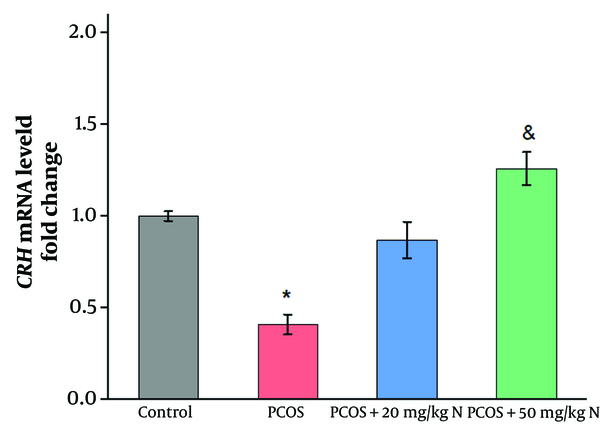
As shown in Figures 2 and 3, the induction of PCOS significantly reduced the mRNA levels of nesfatin-1 and CRH compared to the control group (P ≤ 0.05). Administration of 20 mg/kg naringenin did not result in a significant increase in the mRNA levels of nesfatin-1and CRH compared to the PCOS group. However, the injection of 50 mg/kg naringenin significantly increased the mRNA levels of nesfatin-1 and CRH compared to the PCOS group (P ≤ 0.05). Additionally, a significant difference was observed between the effects of 20 mg/kg and 50 mg/kg naringenin on the mRNA levels of nesfatin-1 (P ≤ 0.05).
5. Discussion
The consumption of herbal remedies has a positive association with the reduction of infertility problems. Previous studies have investigated the effect of naringenin on managing amenorrhea and abnormal ovulation. In the current study, CRH gene expression decreased in the PCOS group compared to the control. However, CRH mRNA levels returned to the baseline level of intact rats following naringenin injection for two weeks. Previous studies also indicate a decrease in CRH levels in PCOS patients compared to intact rats, supporting the present findings (11). Corticotropin-releasing hormone is synthesized in the hypothalamic paraventricular nucleus (PVN) and has various physiological functions, including the regulation of behavior, nutrition, reproduction, and GnRH neuron function (12). Evidence suggests that CRH is involved in steroid biosynthesis, inflammatory processes, ovulation, and luteolysis (13). In PCOS patients, the decrease in CRH gene expression may be due to increased androgen levels. The CRH neurons express androgen receptors (ARs), indicating that these neurons are targets for androgen action. The suppressive effects of androgens on CRH may occur directly by binding to ARs on CRH neurons in the PVN, or androgens may act on CRH neurons indirectly via the bed nucleus of the stria terminalis (BNST), the medial preoptic area (mPOA), the suprachiasmatic nucleus (SCN), and the arcuate nucleus (ARC). The PVN receives afferent signals from these areas, which play a crucial role in regulating the HPA axis response (14).
In PCOS, the reduction of CRH may also be due to the effect of androgens on ghrelin. Androgen levels have an inverse relationship with ghrelin, as demonstrated by the increase in ghrelin levels following the injection of the anti-androgen flutamide (15). Ghrelin has a direct relationship with CRH neuron activity (16). This peptide is synthesized not only in gastric mucosal cells but also in specific populations of neurons within the central nervous system, including the hippocampus, hypothalamus, midbrain, and spinal cord (17). Research indicates that ghrelin’s effect on GnRH secretion is mediated by the ghrelin receptor (GHS-R) located on GnRH neurons. When a ghrelin receptor antagonist is administered, it blocks the inhibitory effect of ghrelin on GnRH secretion (18). Additionally, women with PCOS have been reported to have lower ghrelin levels (19). Evidence suggests that ghrelin, by stimulating CRH neuron activity, promotes the release of CRH, adrenocorticotropic hormone (ACTH), and corticosteroids (16). Furthermore, previous findings indicate that naringenin enhances ghrelin receptor activity (20). Therefore, it is hypothesized that naringenin may contribute to the restoration of CRH levels to baseline in the PCOS model by stimulating the ghrelin receptor on CRH neurons.
Estrogen, a critical sex hormone in the reproductive axis, supports follicular growth and the secretion of gonadotropins from the pituitary gland. Estrogen acts through two receptors: the alpha receptor (ERα) and the beta receptor (ERβ). Studies show that estrogen levels decrease in PCOS due to the inhibition of aromatase activity, which is responsible for converting androgens to estrogen. This process is essential as estrogen plays a central role in ovarian function by regulating androgen production (21, 22). There is also an interaction between estrogen and CRH in reproductive regulation, as estrogen receptors are located on CRH neurons, and CRH is stimulated by both ERα and ERβ (23). Additionally, studies reveal that naringenin has structural similarities to beta-estradiol (24) and can bind to both alpha and beta receptors (25). It has also been reported that naringenin stimulates aromatase activity, potentially leading to an increase in estrogen levels (26). Thus, it is likely that naringenin increases CRH mRNA to baseline in the PCOS rat model by enhancing aromatase activity and estrogen receptor function.
In the present study, the effect of naringenin on hypothalamic gene expression of nesfatin-1 was investigated in a rat model of estradiol valerate-induced PCOS. Results showed a significant decrease in nesfatin-1 gene expression in the PCOS group compared to the control group, consistent with previous studies (27). Several studies in rats indicate the presence of nesfatin-1 and its co-expression with other transmitters in the brain, particularly in hypothalamic nuclei such as the PVN and ARC (28). Evidence suggests that nesfatin-1 can regulate reproductive function by acting directly on GnRH neurons (29). Research indicates a positive relationship between nesfatin-1 and levels of follicle-stimulating hormone (FSH), estrogen, and progesterone. Low levels of nesfatin-1 in PCOS impair follicular growth by inhibiting FSH (30).
One of the primary metabolic abnormalities associated with PCOS is insulin resistance (IR), which plays a significant role in the condition's pathophysiology (31). Many women with PCOS are overweight or obese, which exacerbates impaired insulin action (31). Elevated insulin levels can further reduce nesfatin-1 levels in PCOS (32). As a natural compound, naringenin improves insulin sensitivity and glucose metabolism (33). In this study, naringenin administration increased hypothalamic gene expression of nesfatin-1 in the PCOS model rats. The enhancing effect of naringenin on nesfatin-1 may be mediated by its role in glucose homeostasis.
There is also a close relationship between the serotonergic system and nesfatin-1. Serotonin inputs in the hypothalamus interact with nesfatin-1 neurons, with serotonin able to stimulate nesfatin-1 production. For example, a study reported that administering a serotonin receptor agonist to mice increased nesfatin-1 levels (34). Furthermore, previous research has shown that naringenin may influence serotonin system function, increasing serotonin levels in rats receiving naringenin (35). This suggests that naringenin may upregulate hypothalamic nesfatin-1 expression by enhancing serotonergic system activity in the PCOS rat model.
5.1. Conclusions
In summary, naringenin increased the expression of CRH and nesfatin-1 genes in the hypothalamus of PCOS rats. This study contributes to understanding the molecular mechanisms of naringenin in the reproductive system. Thus, naringenin, as a natural phytoestrogen, may help improve HPG axis function in PCOS.