1. Background
In recent decades, numerous studies have confirmed a decline in semen quality among men, contributing to decreased male fertility worldwide (1). This decline in sperm quality can result from improper diets, increased obesity, and exposure to environmental and occupational pollutants (2). Environmental factors, including pesticides, external estrogens, and heavy metals, have been shown to adversely affect male fertility (3).
Heavy metals are among the most significant and serious environmental pollutants contributing to reproductive dysfunction (4, 5). Humans are exposed to metal particles such as lead, cadmium, and arsenic through occupational and environmental sources (3). These metals naturally exist in the earth's crust and are introduced into the environment through natural forces. However, industrial activities have significantly increased their release, resulting in greater exposure to these metals via food, water, and air pollution (6).
High concentrations of heavy metals have been linked to impaired sperm quality (7). Exposure to lead is associated with abnormalities in sperm motility (asthenozoospermia), morphology (teratozoospermia), and DNA integrity (8-11). Cadmium, another highly toxic heavy metal, is found in various industrial products and contaminated drinking water (12). Exposure to cadmium is negatively associated with sperm concentration, motility, and morphology, adversely affecting male fertility (13, 14). Similarly, arsenic exposure has been implicated in reducing sperm count, motility, and normal morphology (15-17). Although the exact mechanism of toxicity remains poorly understood, heavy metals are known to cause cellular damage through the production of reactive oxygen species (ROS) (18).
Ahvaz, the capital of Khuzestan Province, is a metropolitan city in southwest Iran, bordering Iraq, Kuwait, and Saudi Arabia. With coordinates of 31°20'N and 48°40'E, a surface area of 528 km², and a population of 1.112 million, Ahvaz is one of Iran's major industrial cities (19). The city's air pollution is exacerbated by the presence of numerous oil, gas, petrochemical, metal, and non-metal industries. Furthermore, dust pollution is another significant issue in Ahvaz, primarily attributed to the city's proximity to western deserts and fine dust sources in neighboring countries such as Iraq and Saudi Arabia (1).
2. Objectives
The present study aimed to investigate the seminal levels of lead, cadmium, and arsenic, as well as oxidative status, in men residing in Ahvaz, southwest Iran.
3. Methods
3.1. Subjects
This study was approved by the Ethics Committee of Shahid Chamran University of Ahvaz (EE/1400.2.24.25679/scu.ac.ir). The nature and purpose of the study were explained to all participants, and written informed consent was obtained from each of them. A total of 100 men attending the Narges Medical Genetics and Prenatal Diagnosis Laboratory in Ahvaz, southwest Iran, from April 2021 to May 2022, were recruited for semen analysis. The sample size was determined based on related studies (20, 21).
Smokers, drug addicts, alcohol consumers, and men with a history of serious systemic diseases, chronic illnesses, or male reproductive disorders such as sexually transmitted diseases, prostatitis, varicocele, urinary tract infections, cryptorchidism, or previous genital surgery were excluded from the study (22).
3.2. Semen Collection
After collecting semen samples in wide-mouth sterile containers, semen analysis was conducted 30 minutes after liquefaction at room temperature, following the guidelines of the World Health Organization (WHO) (23). The semen samples were centrifuged at 15,000 g for 15 minutes. The supernatant was separated and used as seminal plasma, which was then frozen at -20°C until further analysis.
3.3. Semen Analysis
A trained technician analyzed sperm concentration, motility, and morphology in duplicate samples. Subjects were considered normal if they met the following criteria: ≥ 39 × 10⁶ total sperm count, ≥ 15 × 10⁶ sperm count per mL, ≥ 40% total motile sperm (progressive and non-progressive), ≥ 32% progressive motility, ≥ 4% normal morphology, and < 1 × 10⁶ WBC/mL.
An improved Neubauer hemocytometer (Hamburg, Germany) was used to determine sperm concentration. The total sperm count was calculated by multiplying the semen volume by the sperm concentration. The motility of at least 200 sperm was assessed and classified into progressive motile, non-progressive motile, and immotile categories, after which total sperm motility was calculated.
For sperm morphology assessment, semen smears were stained using the Papanicolaou method, and at least 200 sperm were evaluated at 1000× magnification according to WHO guidelines (23).
3.4. Seminal Heavy Metal (Lead, Cadmium and Arsenic) Analysis
Semen samples were analyzed to determine the levels of lead, cadmium, and arsenic in seminal plasma. A flame atomic absorption spectrophotometer (Thermo Fischer, USA) was used to measure the concentrations of these heavy metals in the seminal plasma.
3.5. Seminal Lipid Peroxidation and Antioxidant Enzymes Activity Analysis
Lipid peroxidation levels and antioxidant enzyme activities were analyzed in seminal plasma. Malondialdehyde (MDA) levels, as an indicator of lipid peroxidation, were measured using the thiobarbituric acid test at 532 nm and expressed as nmol/mL of seminal plasma. Seminal glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities were evaluated using colorimetric assay kits (Randox Ltd., United Kingdom).
3.6. Statistical Analysis
SPSS version 16 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Differences between normozoospermic and asthenozoospermic men were assessed using the non-parametric Mann-Whitney test. Correlations between various parameters were evaluated using the Spearman test. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Semen Analysis
Subjects were divided into normozoospermic and asthenozoospermic groups based on their semen quality. Semen characteristics for normozoospermic and asthenozoospermic individuals are presented in Table 1. Significant differences were observed in semen volume, sperm concentration, total motility (progressive and non-progressive), and normal morphology between patients and healthy individuals (Table 1).
| Parameters | Normozoospermic (n = 58) | Asthnozoospermic (n = 42) | P-Value |
|---|---|---|---|
| Age (y) | 33.0 (29.0 - 38.2) | 34.0 (30.0 - 38.5) | 0.997 |
| BMI (kg/m2) | 23.3 (22.3 - 23.8) | 23.1 (22.1 - 23.6) | 0.234 |
| Semen volume (mL) | 3.0 (2.5 - 4.0) | 2.5 (2.5 - 3.5) | 0.049 |
| Sperm count (million/mL) | 30.0 (29.5 - 39.2) | 25.0 (18.0 - 35.0) | < 0.001 |
| Total motility (%) | 49.5 (45.0 - 55.0) | 32.5 (28.0 - 38.0) | < 0.001 |
| Normal morphology (%) | 8.0 (6.5 - 12.0) | 5.0 (4.0 - 8.0) | 0.001 |
Abbreviation: BMI: Body Mass Index.
a Results expressed as median values and interquartile ranges.
b Mann-Whitney U-test was done as the test of significant.
c The level of significance was set at P < 0.05.
4.2. Seminal Heavy Metal (Lead, Cadmium and Arsenic) Analysis
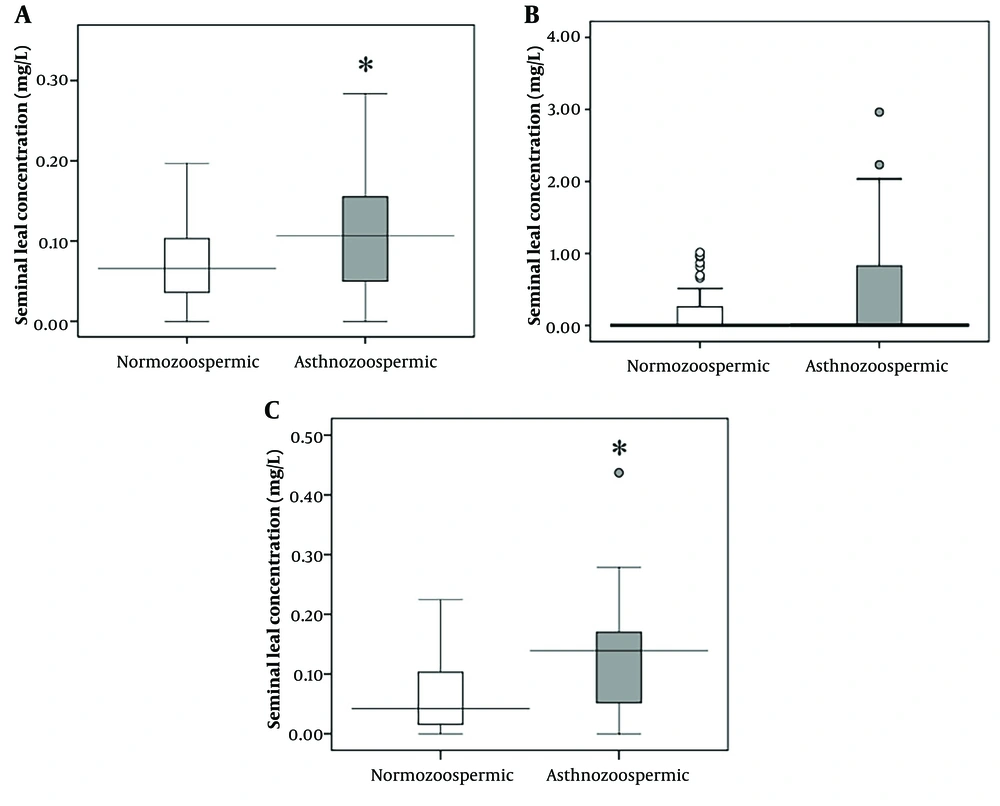
Seminal concentrations of lead were significantly higher (P = 0.015) in asthenozoospermic patients compared to normozoospermic individuals (Figure 1A). Significant negative relationships were observed between seminal lead concentrations and sperm count (r = -0.360, P < 0.001), motility (r = -0.257, P = 0.010), and normal morphology (r = -0.300, P = 0.002) (Table 2).
| Parameters | Lead (mg/L) | Cadmium (mg/L) | Arsenic (mg/L) |
|---|---|---|---|
| Semen volume (mL) | r = 0.089, P = 0.379 | r = 0.065, P = 0.410 | r = -0.113, P = 0.261 |
| Sperm count (million/mL) | r = -0.360, P < 0.001 | r = -0.461, P < 0.001 | r = -0.211, P = 0.035 |
| Total motility (%) | r = -0.257, P = 0.010 | r = -0.432, P < 0.001 | r = -0.285, P = 0.004 |
| Normal morphology (%) | r = -0.300, P = 0.002 | r = -0.476, P < 0.001 | r = -0.219, P = 0.028 |
a The level of significance was set at P < 0.05.
Additionally, significantly higher levels of cadmium (P < 0.001) were detected in the semen of asthenozoospermic men compared to normozoospermic individuals (Figure 1B). Seminal cadmium levels were negatively associated with sperm count (r = -0.461, P < 0.001), motility (r= -0.432, P < 0.001), and normal morphology (r = -0.476, P < 0.001) (Table 2).
However, there was no significant difference (P = 0.457) in seminal arsenic levels between asthenozoospermic men and normozoospermic individuals (Figure 1C). Despite this, a significant negative relationship was identified between arsenic concentration and sperm count (r = -0.211, P = 0.035), motility (r = -0.285, P = 0.004), and normal morphology (r = -0.219, P = 0.028) (Table 2).
4.3. Seminal Lipid Peroxidation and Antioxidant Enzymes Activity Analysis
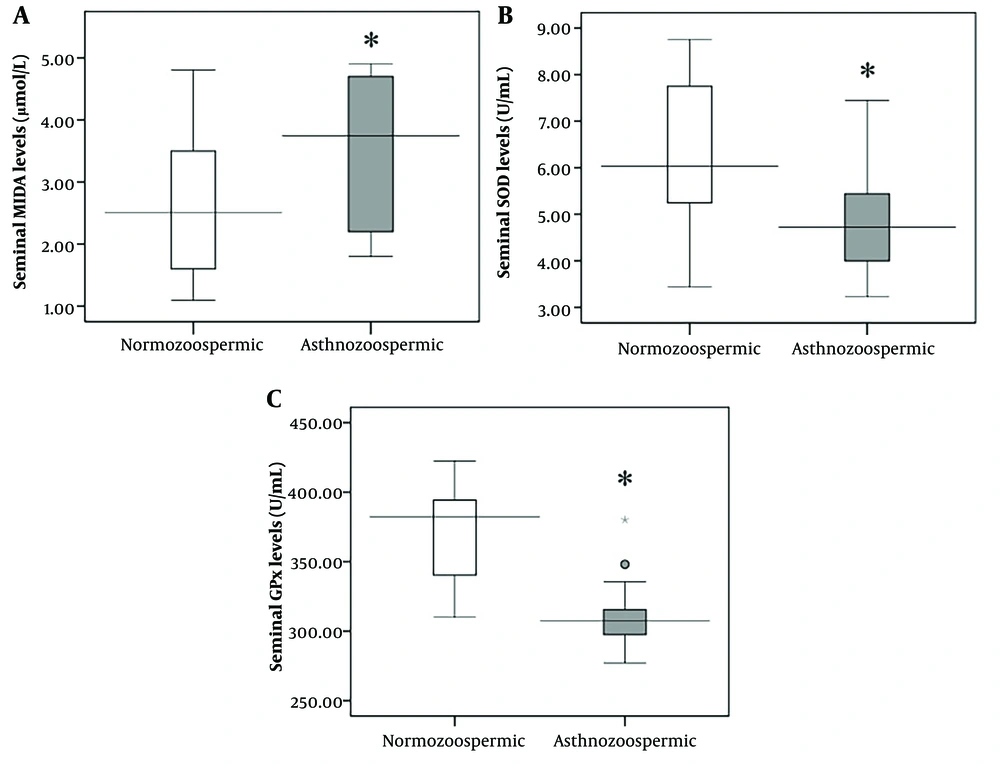
Significantly higher seminal levels of MDA (P < 0.001) and lower activity of SOD and GPx enzymes were observed in asthenozoospermic men compared to normozoospermic men (Figure 2A - C). Significant associations were identified between sperm count, total motility, normal morphology, and seminal MDA levels, as well as SOD and GPx enzyme activity (Table 3).
A - C, seminal levels of lipid peroxidation and antioxidant enzymes activity in normozoospermic (n = 58) and asthnozoospermic (n = 42) men in Ahvaz, southwest Iran. MDA, malondialdehyde; GPx, glutathione peroxidase; SOD, superoxide dismutase. Mann-Whitney U-test was done as the test of significant and the results expressed as median. *The level of significance was set at P < 0.05.
| Parameters | MDA (nmol/mL) | SOD (U/mL) | GPx (mU/mL) |
|---|---|---|---|
| Semen volume (mL) | r = -0.082, P = 0.417 | r = 0.090, P = 0.375 | r = 0.150, P = 0.137 |
| Sperm count (million/mL) | r = -0.472, P < 0.001 | r = 0.481, P < 0.001 | r = 0.479, P < 0.001 |
| Total motility (%) | r = -0.422, P < 0.001 | r = 0.490, P < 0.001 | r = 0.740, P < 0.001 |
| Normal morphology (%) | r = -0.430, P < 0.001 | r = 0.398, P < 0.001 | r = 0.401, P < 0.001 |
Abbreviations: MDA, malondialdehyde; GPx, glutathione peroxidase; SOD, superoxide dismutase.
a The level of significance was set at P < 0.05.
A significant correlation was observed between seminal lead concentration and seminal MDA levels (r = 0.339, P = 0.001), as well as SOD (r = -0.383, P < 0.001) and GPx (r = -0.372, P < 0.001) enzyme activity (Table 4). Seminal cadmium levels were significantly associated with seminal MDA levels (r = 0.545, P < 0.001) and SOD (r = -0.623, P < 0.001) and GPx (r = -0.586, P < 0.001) enzyme activity (Table 4).
| Parameters | Lead (mg/L) | Cadmium (mg/L) | Arsenic (mg/L) |
|---|---|---|---|
| MDA (nmol/mL) | r = 0.339, P = 0.001 | r = 0.545, P < 0.001 | r = 0.183, P = 0.068 |
| SOD (U/mL) | r = -0.383, P < 0.001 | r = -0.623, P < 0.001 | r = -0.142, P = 0.159 |
| GPx (mU/mL) | r = -0.372, P <0.001 | r = -0.586, P < 0.001 | r = -0.125, P = 0.215 |
Abbreviations: MDA, malondialdehyde; SOD, superoxide dismutase; GPx, glutathione peroxidase.
a The level of significance was set at P < 0.05.
No significant associations were found between seminal arsenic concentration and seminal MDA levels or the activity of SOD and GPx enzymes (Table 4).
5. Discussion
With the expansion of industrial activities worldwide, pollution caused by heavy metals has significantly impacted human health (24). Ahvaz has been reported as one of the most polluted cities in Iran and globally, with air pollution adversely affecting the health of its residents (25). In recent years, the industrial development of Ahvaz, combined with population growth and increased vehicle traffic, has contributed to the deposition of heavy metals in dust particles (26). Additionally, dust is a significant air pollutant in Ahvaz, due to its proximity to deserts and dust sources in neighboring countries (1). The intensive activity of oil, gas, and metal industries, along with light and heavy traffic, has resulted in higher-than-background levels of heavy metals such as lead, zinc, copper, chromium, cadmium, and arsenic in the surface soils of Ahvaz (27).
The present study evaluated seminal levels of heavy metals, including lead, cadmium, and arsenic, as well as oxidative status, and investigated their relationships with sperm quality in men from Ahvaz, southwest Iran. Our findings revealed higher seminal levels of lead in asthenozoospermic men compared to normozoospermic individuals. A significant negative relationship was observed between lead concentration and sperm count, motility, and normal morphology. Higher seminal lead concentrations were also associated with decreased activity of seminal antioxidant enzymes and increased levels of lipid peroxidation.
Nandi et al. reported that lead levels in the seminal plasma of normozoospermic individuals were lower compared to infertile men (28). Similarly, Famurewa and Ugwuja found that lead levels in the seminal plasma of azoospermic and oligozoospermic men were higher than in normozoospermic individuals, correlating with unfavorable decreases in parameters such as sperm concentration, motility, and normal morphology (29).
According to the findings of Tanricut et al. , exposure to low levels of environmental lead alters semen quality and affects sperm chromatin density (30). Similarly, Pant et al. observed a significant negative relationship between lead concentration and sperm motility and concentration in infertile individuals compared to normozoospermic men (15). In this context, an analysis of semen quality in battery factory workers revealed high levels of lead in their semen, which was associated with reduced sperm concentration (31). However, Plechaty et al. found no relationship between sperm concentration, motility, and lead concentration in South African battery workers (32).
Additionally, higher levels of cadmium were observed in the semen of asthenozoospermic men compared to normozoospermic individuals. Seminal cadmium levels were negatively associated with sperm count, motility, and normal morphology. Higher concentrations of seminal cadmium were also linked to decreased activity of seminal antioxidant enzymes and increased levels of lipid peroxidation. Nandi et al. reported that cadmium significantly reduces seminal fluid volume (28). Famurewa and Ugwuja found that cadmium adversely affects sperm concentration, motility, and normal morphology (29). Zhao et al. demonstrated that cadmium decreases the total percentage of motile sperm in humans and mice in a dose- and time-dependent manner, reducing sperm motility and impairing sperm penetration into the egg, as well as embryo development (12). It has also been shown that cadmium induces inflammation in the testes of adult mice, leading to a decline in sperm parameters (33).
However, Keck et al. and Meeker et al. found no correlation between seminal cadmium levels and sperm parameters (34, 35). Xu et al. reported that cadmium adversely affects the prostate gland, which secretes seminal plasma, resulting in an inverse relationship between cadmium concentration and seminal fluid volume (7).
Our data indicated higher seminal levels of arsenic in asthenozoospermic men compared to normozoospermic individuals. A significant negative relationship was observed between arsenic concentration and sperm count and motility. Higher concentrations of seminal arsenic were also associated with decreased activity of seminal antioxidant enzymes and increased levels of lipid peroxidation. Nandi et al. reported that individuals with high arsenic concentrations had significantly higher percentages of sperm with abnormal motility (28). Similarly, a study conducted on men referred to infertility clinics in Michigan, United States, found that smoking men had a significantly increased risk of low sperm motility due to prolonged arsenic exposure (35). Li et al. demonstrated that arsenic disrupted spermatogenesis in mice, leading to a decline in sperm quality (36).
Heavy metals are known to damage cells by inducing oxidative stress through the production of ROS (37). In this study, higher levels of lipid peroxidation and lower antioxidant enzyme activity were observed in asthenozoospermic men compared to normozoospermic individuals. Significant negative relationships were identified between seminal concentrations of lead, arsenic, and cadmium and the activity of antioxidant enzymes. Reduced antioxidant enzyme activity and increased lipid peroxidation, resulting from excessive ROS production, have been linked to impaired spermatogenesis (38).
Lead exposure induces testicular oxidative stress by increasing MDA levels and reducing the activity of antioxidant enzymes such as SOD, GPx, and catalase, thereby decreasing sperm count and motility (39). Similarly, cadmium exposure has been associated with excessive ROS production and oxidative stress. Leite et al. reported that even environmentally realistic doses of cadmium reduced testicular antioxidant status in Wistar rats (40). Sperm motility is impaired by excessive ROS production, which decreases axonemal protein phosphorylation and/or adenosine triphosphate levels and increases membrane lipid peroxidation (41).
Additionally, arsenic exposure has been linked to impaired spermatogenesis, reduced fertilization ability, and decreased sperm count and progressive motility. Like other heavy metals, arsenic exposure increases ROS and MDA levels while reducing the activity of glutathione (GSH) and SOD in the testes (42).
5.1. Conclusions
In conclusion, the present study demonstrates that higher levels of seminal heavy metals, along with decreased antioxidant enzyme activity and increased lipid peroxidation, were observed in asthenozoospermic men in Ahvaz, southwest Iran. These findings suggest that heavy metals adversely affect semen quality by inducing oxidative stress. Further studies with larger sample sizes are required to validate these findings and provide more comprehensive insights.