1. Background
Salmonella typhimurium is commonly transmitted to humans through the fecal-oral route. In humans, its primary colonization sites are the ileum, liver, spleen, gallbladder, and blood (1-3). This bacterium is a major cause of bacteremia, septicemia, typhoid fever, and diarrhea (4).
Complications of typhoid fever include leukopenia, thrombocytopenia, and elevated levels of C-reactive protein (CRP) and alanine aminotransferase (ALT) (5, 6). Contaminated water and poor socioeconomic conditions are key factors contributing to the spread of enteric fever (6, 7). In 2019, Pakistan introduced the typhoid conjugate vaccine, approved by the World Health Organization, to combat typhoid fever (8, 9). The vaccine's results were highly effective in reducing infection rates (10).
Until the 1970s, antibiotics such as ampicillin, chloramphenicol, and cotrimoxazole (trimethoprim-sulfamethoxazole) were commonly used to treat S. typhimurium infections. Unfortunately, widespread bacterial drug resistance has necessitated the development of new treatments (9, 11-15).
Nannorrhops ritchiana (Griff) Aitch is a species native to Pakistan, Afghanistan, and Iran. This versatile and resilient shrub thrives under extreme environmental conditions, such as strong winds, extreme temperatures, and water scarcity (16). This plant has been commonly used in different countries (17-31). Historically, the leaves and stems of N. ritchiana were used to make mats, fences, and house roofs (32, 33). The dried leaves, stems, and petioles of the Mezri palm have also been used as household fuel. In southern Europe and subtropical regions of the Americas, N. ritchiana is cultivated as an ornamental plant (34).
Temple figs, belonging to the Ficus genus and the Moraceae family, are perennial, evergreen plants (35). Among the therapeutic properties of temple figs are their antibacterial, antiviral, and antifungal activities, as well as acetylcholinesterase inhibition. Extractive compounds from this plant have applications in treating skin diseases (inflammation, swelling, and wound healing), breast cancer, vaginal diseases, digestive and respiratory disorders, epilepsy, nervous system issues, and regulating the menstrual cycle (36, 37).
Ducrosia anethifolia, native to Iran and other parts of the Middle East, has essential oils (DaEO) containing bioactive compounds such as simene. These oils exhibit antimicrobial activity against various bacteria and fungi (38, 39). Studies suggest that combining essential oils with complementary antimicrobial properties can create stronger preservation methods compared to using individual oils alone (40-43).
The Capparis spinosa plant, from the Capparidaceae family, is native to the Mediterranean basin and is widely found in Iran (44, 45). This plant contains compounds that contribute to disease prevention and play a role in reducing cancer incidence (46).
2. Methods
The plants used in this research were collected from the plains of Sistan and Baluchistan. Forty grams of dry plant powder were crushed and placed in half-liter jars containing 200 mL of 96% ethanol. These jars were placed on a shaker for 24 hours, then filtered, and the extract was stored in a refrigerator at 4°C.
2.1. Isolation of Bacteria
Stool samples were collected from Zabol city and kept at room temperature for up to 6 hours immediately after collection. The samples were then transferred to selective culture media, including Salmonella-Shigella agar and Simon sulfite agar, and incubated at 37°C for 24 hours. Twelve strains of S. typhimurium were subsequently isolated and identified using differential cultures. The diameter of the inhibition zone of the plant extract was determined using the well diffusion method against S. typhimurium.
3. Results
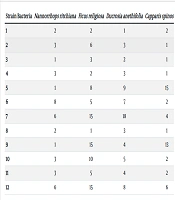
The results of the study showed that the maximum diameter of the inhibition halo for the Doz ethanolic extract was 8 mm, while the minimum diameter was 1 mm. For fig leaves, the maximum and minimum inhibition diameters were 15 mm and 2 mm, respectively. The musk extract exhibited inhibition diameters ranging from a minimum of 1 mm to a maximum of 18 mm. Additionally, the inhibition diameter for snake grass fruits was recorded as 15 mm (Table 1).
| Strain Bacteria | Nannorrhops ritchiana | Ficus religiosa | Ducrosia anethifolia | Capparis spinose L. Fruit |
|---|---|---|---|---|
| 1 | 2 | 2 | 1 | 2 |
| 2 | 3 | 6 | 3 | 1 |
| 3 | 1 | 3 | 2 | 1 |
| 4 | 3 | 2 | 3 | 1 |
| 5 | 1 | 8 | 9 | 15 |
| 6 | 8 | 5 | 7 | 2 |
| 7 | 6 | 15 | 18 | 4 |
| 8 | 2 | 1 | 3 | 1 |
| 9 | 1 | 15 | 4 | 13 |
| 10 | 3 | 10 | 5 | 2 |
| 11 | 3 | 5 | 4 | 2 |
| 12 | 6 | 15 | 8 | 6 |
4. Discussion
Many studies have demonstrated a direct relationship between the phenolic and flavonoid content of different plant organs and their antioxidant potential.
In one study investigating the antimicrobial and antifungal effects of Nannorrhops ritchiana leaves, the maximum diameter of the inhibitory halo was observed at a concentration of 300 µg/mL (34). Additionally, the ethanolic fraction of the aerial parts of Nannorrhops ritchiana exhibited significant antifungal activity against Candida albicans, Aspergillus niger, and Microsporum canis, along with moderate inhibition (56%) against Staphylococcus aureus (47). Another study showed that this plant demonstrated good antibacterial effects against Proteus mirabilis, Shigella flexneria, Escherichia coli, and Staphylococcus aureus (48).
In the study by Daing et al., the minimum inhibitory concentration (MIC) for Escherichia coli, Staphylococcus aureus, Salmonella enterica, and Enterococcus faecalis strains was found to be 6.67, 4.17, 5, and 80 mg/mL, respectively (49). Similarly, in the study by Tkachenko et al., the halo created by fig leaf extract in 200 and 400 µl plates of bacterial suspension measured 8.83 mm and 13.42 mm, respectively (50).
A study exploring changes in Ducrosia anethifolia ethanolic extract reported the highest levels of total phenols (148 ± 1.7 mg gallic acid equivalent (GAE) per gram of dry weight) and flavonoids (1.5 ± 97 mg quercetin equivalent (QE) per gram dry weight), along with notable antioxidant, antibacterial, and anti-inflammatory activities (51).
In Almuhanna's study, the methanolic extract of Ducrosia anethifolia demonstrated efficacy against biofilms of methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa on cut wounds in diabetic rats. The extract was more effective against MRSA than Pseudomonas aeruginosa in both in vitro and in vivo tests (52). GC-MS analysis of the ethyl acetate fraction of the methanolic extract revealed key compounds such as 8-ethoxy psoralen (6.5%), prongnin (6.26%), isoaromadendrene epoxide (7.5%), aromadendrene oxide (0.96%), and acid methyl ester (0.46%) (53).
In Obaro et al.'s study, the anti-inflammatory properties of palm oil (PO) and the synergistic effects of an aqueous extract polyherbal formulation (AEPHF) containing Zingiber officinale, Curcuma longa, and Allium sativum were evaluated. The results indicated a significant reduction in paw diameter in the treatment groups compared to the control group (P < 0.01) (54).
Eslammanesh's research investigated the relationship among phenol and flavonoid content, antioxidant properties, and antimicrobial activity of methanolic extracts from nine medicinal plants. The results showed that the methanol extract of Nerium oleander contained the highest phenolic content (3.36 mg/g), while Calotropis procera had the lowest (0.48 mg/g). Furthermore, C. procera extract exhibited the most effective antioxidant properties (85.54 mg/mL), whereas Malva sylvestris extract demonstrated the lowest role in inhibiting free radicals (21.80 mg/mL) (55).
4.1. Conclusions
The results of the study showed that medicinal plants with good antimicrobial properties can play a good role in the treatment and control of salmonella bacteria.