1. Context
Post-transcriptional regulation of genes is primarily mediated by microRNAs (miRNAs), small non-coding RNAs that play a key role in controlling gene expression. Approximately 2,000 miRNAs have been identified in humans, and these molecules are highly conserved across eukaryotic organisms (1, 2). Due to their stability and presence in bodily fluids, miRNAs are increasingly recognized as valuable biomarkers for various diseases. One such miRNA, miR-373, is involved in several essential biological processes, including the regulation of the cell cycle, apoptosis, migration, and stem cell dynamics. MiR-373 is critical in the initiation, progression, invasion, and metastasis of cancer. In the context of colorectal cancer (CRC), the tumor suppressor gene programmed cell death 4 (PDCD4) is negatively regulated by miR-373, with elevated expression levels of miR-373 observed in CRC tissues and cell lines (3-5).
2. Objectives
The primary aim of this review is to explore the role of miR-373 in CRC pathogenesis, focusing on how it regulates key target genes associated with CRC initiation, progression, and metastasis. This review seeks to elucidate the molecular mechanisms by which miR-373 influences inflammatory pathways and carcinogenesis in CRC, and to identify potential therapeutic targets for CRC-specific treatments. Such insights could pave the way for the development of novel diagnostic and therapeutic strategies for CRC (2, 5, 6).
3. Methods
This review article investigates the role of miR-373 in the development and progression of CRC. A systematic search of the literature was conducted using major databases such as PubMed, Scopus, and Google Scholar to identify pertinent studies. The search terms included miR-373, CRC, oncogene, tumor suppressor genes, PTEN, TP53INP1, PI3K-Akt signaling pathway, and cancer progression. Studies included in the review focused on miR-373 expression, its regulatory roles, interactions with tumor suppressor genes, and its impact on oncogenic pathways within CRC. Only studies addressing the role of miR-373 in cancer progression, metastasis, and resistance to therapy were considered relevant. The review encompasses articles published from 2000 to 2025. A qualitative analysis was performed by synthesizing findings from various studies and extracting key information from primary research articles.
4. Results
MiR-373 is commonly overexpressed in CRC tissues and plays a significant role in tumor progression by regulating vital cellular processes such as cell growth, migration, and invasion. It suppresses key tumor suppressor genes like PTEN and TP53INP1, which are involved in apoptosis, DNA repair, and maintaining genomic integrity. This downregulation leads to unchecked cell proliferation, reduced cell death, and enhanced resistance to apoptosis, all of which contribute to the aggressiveness of CRC. Higher levels of miR-373 are linked to advanced stages of the disease and metastasis, positioning it as a potential prognostic marker. Additionally, its involvement in the PI3K-Akt pathway further underscores its contribution to CRC development, making miR-373 a valuable candidate for both diagnostic and therapeutic approaches.
5. Discussion
5.1. Colorectal Cancer
Globally, cancer is the most prevalent illness, with CRC ranking third in terms of fatality (2, 5, 7). The CRC is the third most lethal malignancy worldwide, according to several studies (8). There is strong evidence that regions such as North America and Europe may be able to reduce CRC mortality and morbidity (5, 6, 8, 9). Routine screening requires early diagnosis of precancerous polyps before malignant transformation, as CRC typically takes 10 to 15 years to develop (5, 10). The development and progression of CRC are influenced by a multitude of factors (5, 7). All malignancies, including CRC, are primarily influenced by genetics. Genetic mutations and changes in protein expression drive cancer initiation, development, and invasion. Colorectal cancer advancement and suppression have also been linked to other factors, including miRNAs (5-8). Early detection of CRC can reduce mortality, but despite advancements in diagnostic methods, many CRC patients are diagnosed at an advanced stage. Addressing this issue requires an understanding of the molecular mechanisms underlying CRC development. These mechanisms often involve dysregulated miRNA expression, aberrant DNA methylation patterns, and mutations in key genes. Therefore, the purpose of this study is to clarify the anti-cancer and carcinogenic roles of various miRNA types in CRC (2, 5-9).
5.2. MicroRNAs Structure and Biogenesis
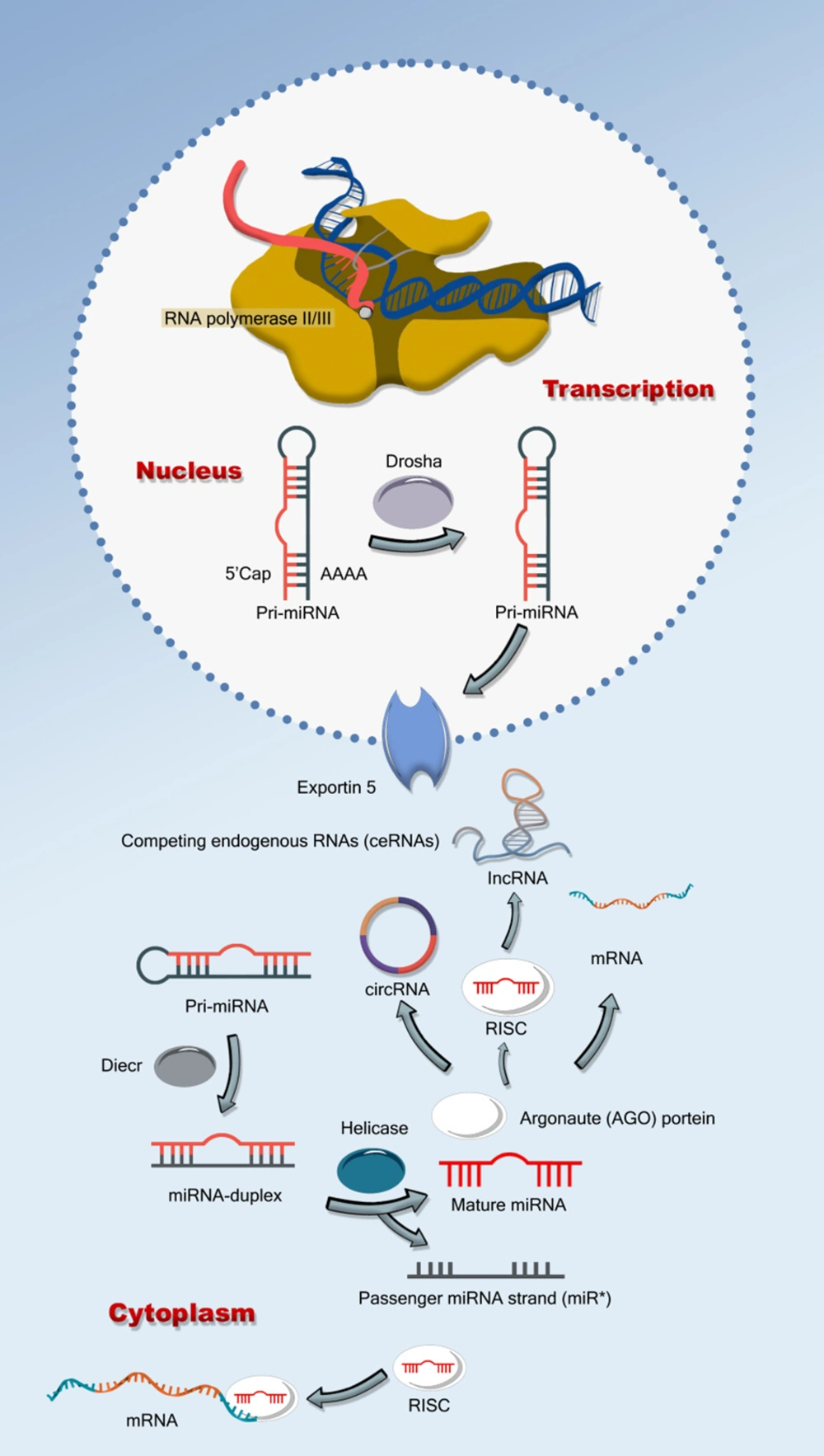
Between 1% and 5% of the human genome is made up of protein-encoding genes, of which at least 30% can be regulated by miRNA (2, 5-7). There are now about 940 distinct miRNA molecules known to exist in the human genome (2, 5, 6, 8). It is evident that miRNA is crucial for controlling gene expression, which governs a number of cellular and metabolic processes, even if there is still much to learn about the specific targets and biological functions of miRNA molecules (1, 2, 5). Single-stranded, non-coding, small, evolutionarily conserved RNA molecules known as miRNAs connect to their target miRNA in one of two ways to stop proteins from forming (1, 2, 4, 5, 8). Mature miRNA, which enters the effector complex known as the RNA induced silencing complex (RISC), is created by the double cleavage of primary miRNA (pri-miRNA) the miRNA functions as a guide to negatively regulate the target mRNA's production by base-pairing with it the complementarity of the guide and the target messenger RNA (mRNA) determines the silencing technique, which may be translation inhibition or cleavage followed by destruction (1, 2, 4, 5). Although the overall role of miRNA is well established, less is known about the molecular mechanisms behind miRNA production and gene silencing (5). Even if the biological relevance of the identified miRNAs may not be known, information on their regulation and function may be obtained by looking at their expression patterns (2, 5, 9). These results imply that specific cancers have different miRNA expression patterns, which raises the possibility that miRNA plays a factor in the development of cancer and other illnesses (1, 2, 5, 9, 10). It has been demonstrated that baseline expression profiling is clinically important to cancer diagnosis, progression, and prognosis, despite our limited knowledge of these molecules. (Figure 1) (1, 2, 4-6, 8, 10).
Outlines the biogenesis and function of microRNAs (miRNAs), starting with pri-miRNA transcription, processing by Drosha into pre-miRNA, export by exportin 5, and cleavage by Dicer into mature miRNA. The mature miRNA is then integrated into the RISC complex to regulate gene expression by targeting messenger RNAs (mRNAs), lncRNAs, and circRNAs, while competing endogenous RNAs (ceRNAs) can influence this process by competing for shared miRNAs.
5.3. MiR-373 Functions
A member of the miRNA family, miR-373 is essential for many cellular functions, including those linked to cancer. Like other miRNAs, miR-373 sequences can come from exonic, intronic, or intergenic regions and are found all across the genome. RNA polymerase II starts the transcription of miR-373, which results in the production of initial transcripts called pri-miRNAs. The microprocessor complex, which is made up of the class II ribonuclease III enzyme Drosha and its cofactor DiGeorge syndrome critical region 8 (DGCR8) in the nucleus, cleaves these pri-miRNAs once they take on a hairpin form. Precursor miRNAs, or pre-miRNAs, such as pre-miR-373, are produced by this cleavage (1-5, 10-12). Pre-miRNAs, including pre-miR-373, are produced and then transported to the cytoplasm by Exportin-5 through a GTP-dependent mechanism that involves Ran-GTP. Additionally, exportin-5 shields the pre-miRNA from nuclear deterioration. Dicer, an important endoribonuclease, further processes pre-miR-373 in the cytoplasm, sometimes in conjunction with the TAR RNA-binding protein (TRBP) (12-15). Due to its crucial function in miRNA maturation, the exact management of a miRNA duplex dicer is crucial since this process eliminates the loop structure. Argonaute (Ago) proteins are then used to divide the miRNA duplex of miR-373 into single strands, one of which is then integrated into the RNA-induced silencing complex (RISC). MiR-373 can control gene expression by binding to complementary sites on target mRNAs with the help of the RISC complex (3, 5, 11, 14, 16).
5.4. Biogenesis of MiR-373 in Colorectal Cancer Cell Lines
The biogenesis of miR-373 in CRC cell lines has garnered significant attention due to its critical role in tumor development and progression. MiR-373, a microRNA implicated in diverse cellular processes, is transcribed by RNA polymerase II from its genomic loci, producing a primary transcript known as pri-miR-373 (1-5). This transcript adopts a characteristic hairpin structure, essential for subsequent processing events within the nucleus, the microprocessor complex, comprising Drosha (a class II ribonuclease III enzyme) and its cofactor DGCR8, cleaves pri-miR-373 to generate the precursor molecule pre-miR-373. Exportin-5, in a Ran-GTP-dependent manner, then transports pre-miR-373 to the cytoplasm, safeguarding it from nuclear degradation (1-3, 11, 13, 16). Once in the cytoplasm, Dicer, another RNase III endoribonuclease, processes pre-miR-373 into a mature miRNA duplex with the assistance of the TRBP. One strand of this duplex is subsequently incorporated into the RISC, which includes Ago proteins. This mature miR-373 guides RISC to specific mRNA targets via sequence complementarity, leading to translational repression or mRNA degradation (3, 12, 13, 16, 17).
In the context of CRC, miR-373 exhibits a dual role, acting both as an oncogene and a tumor suppressor depending on the molecular environment as an oncogene, miR-373 promotes epithelial-mesenchymal transition (EMT), a critical process for tumor invasion and metastasis, by targeting tumor suppressor genes and regulatory pathways conversely, miR-373 can also act as a tumor suppressor by downregulating oncogenic factors and enhancing apoptosis these dual roles underscore the complexity of miR-373’s functions in CRC, making it a compelling target for research and therapeutic intervention (1, 4, 5, 16-18). Studies on CRC cell lines reveal that the dysregulation of miR-373 biogenesis or its target interactions can profoundly impact cellular behavior, including proliferation, migration, and resistance to chemotherapy. Further understanding of miR-373’s biogenesis and its downstream effects in CRC could pave the way for novel diagnostic biomarkers and miRNA-based therapies, offering hope for improved patient outcomes in CRC (14, 15, 17, 19, 20).
5.5. The Role of MiR-373 in the Pathogenesis of Colorectal Cancer
MiR-373 plays a pivotal role in the progression and metastasis of CRC by regulating several cellular processes that support tumor growth, survival, and spread. One of the most significant ways miR-373 contributes to CRC is by targeting and suppressing critical tumor suppressor genes, such as LATS2 and P53 these genes usually act as defense mechanisms to halt uncontrolled cell growth and initiate cell death in response to DNA damage. However, by downregulating these tumor suppressors, miR-373 allows cancer cells to evade these crucial checkpoints as a result, the cancer cells can continue to grow unchecked, even when they carry genetic damage, leading to the rapid expansion of the tumor and resistance to conventional therapies that rely on triggering cell death in damaged cells (12, 14, 15, 17, 19, 21).
Beyond promoting unchecked growth, miR-373 also enhances the ability of cancer cells to spread throughout the body, a process called EMT enables cancer cells to lose their attachment to one another and acquire a more mobile, invasive phenotype. MiR-373 promotes EMT by targeting E-cadherin, a protein responsible for maintaining strong cell-cell adhesion when miR-373 reduces E-cadherin expression, it weakens the bonds between cells, making it easier for them to detach from the primary tumor and migrate into surrounding tissues this ability to facilitate cancer cell migration is a key factor in the metastatic spread of CRC, as it allows cancer cells to reach distant organs and form secondary tumors (12, 21-23).
In addition to its role in invasion, miR-373 plays a critical part in sustaining the growth and survival of cancer cells by modulating important signaling pathways. MiR-373 suppresses PTEN, a tumor suppressor gene that normally inhibits the PI3K-AKT signaling pathway, which is responsible for promoting cell growth, survival, and proliferation. By downregulating PTEN, miR-373 keeps the PI3K-AKT pathway constantly active, which supports cancer cell survival and growth, even in stressful conditions like chemotherapy or lack of nutrients. MiR-373 also influences the TGF-β/SMAD pathway by targeting SMAD7, an inhibitor of this pathway. When miR-373 reduces SMAD7, it allows SMAD2 and SMAD3 to become activated, driving the expression of genes that contribute to tumor growth, fibrosis, and metastasis. These effects collectively enhance the aggressiveness of CRC, making it harder to treat and manage (12, 19, 21, 22, 24).
Finally, miR-373 facilitates tumor growth by promoting angiogenesis, the formation of new blood vessels that supply the tumor with essential nutrients and oxygen by targeting VEGF, a key molecule that stimulates the growth of blood vessels, miR-373 helps ensure that the tumor maintains a steady supply of blood, which is crucial for its continued growth and spread. Angiogenesis is particularly important in the metastatic process, as it allows the tumor to expand and infiltrate new tissues through its combined influence on tumor growth, invasion, and blood vessel formation, miR-373 plays an integral role in the progression of CRC understanding how miR-373 regulates these key processes could provide valuable insights into new therapeutic strategies aimed at targeting this microRNA such therapies could potentially halt tumor growth, reduce metastasis, and improve patient outcomes, offering hope for more effective CRC treatments in the future (12, 13, 19-23).
5.6. Assessment of MiR-373 in Colorectal Cancer
MiR-373 has recently gained attention as a potential biomarker in CRC the to its involvement in various cellular processes, including cell growth, apoptosis, and metastasis, all of which are crucial for tumor development this microRNA has shown a significant role in regulating pathways that contribute to cancer progression, particularly in CRC research has revealed that miR-373 is abnormally expressed in CRC tissues, with many studies indicating that its levels are significantly higher in tumor samples compared to normal tissues this upregulation of miR-373 is often associated with poorer prognosis and more advanced stages of CRC, making it a potential marker for monitoring disease progression and evaluating patient outcomes. Additionally, elevated levels of miR-373 have been detected in circulating blood and plasma, suggesting that it could serve as a non-invasive diagnostic tool for CRC this provides a promising alternative to traditional tissue biopsy, offering a less invasive approach for detecting the disease (9, 11, 12, 15, 19, 20, 23, 25-27).
MiR-373 is known to regulate several target genes involved in tumor suppression and metastasis, including the tumor suppressor gene p53, which plays a key role in controlling cell growth and apoptosis. When miR-373 is dysregulated, it may lead to uncontrolled cell division and resistance to cell death, both of which contribute to the development and progression of CRC. Furthermore, miR-373 is involved in the process of EMT, a crucial mechanism in cancer metastasis, highlighting its potential role in the spread of CRC to other parts of the body studies focusing on the diagnostic potential of circulating miR-373 have shown promising results detecting elevated miR-373 levels in the blood of CRC patients could help identify those at higher risk or in the early stages of the disease, leading to earlier intervention and potentially better outcomes moreover, combining miR-373 with other miRNAs may enhance the sensitivity and specificity of CRC screening tests, improving early detection and diagnosis (12, 19, 20, 25-30).
In a study by Eyking et al. in 2016 in Germany, the roles of miR-205 and miR-373 in mucinous adenocarcinoma (MAC) of CRC were investigated. The results showed that these miRNAs were specifically upregulated in mucinous tumors, unlike in CRC without mucinous components. In Caco-2 cell models, miR-205 promoted goblet cell expansion and chemoresistance, while miR-373 induced morphological changes, EMT, and tumor progression. These findings suggest that miR-205 and miR-373 may contribute to the aggressive characteristics of MAC in CRC (14).
In a study Chen et al. in 2024 in China explored the role of miR-373 in CRC, focusing on its impact on cell invasion and metastasis. They found that overexpression of miR-373 in SW-480 cells enhanced their invasive capabilities, while miR-373 knockdown reduced invasion. Proteomic analysis identified key pathways, including MAPK, PI3K-Akt, and FAK, as being involved in these processes. The study also suggested that miR-373 may activate the ERK/MAPK pathway to promote CRC cell migration. However, no significant effects were found on cell proliferation or apoptosis (23).
5.7. Conclusions
Altered expression of miRNAs, particularly miRNA-373, has been linked to several malignancies, including colorectal, pancreatic, prostate, and breast cancers. MiRNAs are crucial for cellular processes that contribute to cancer development, such as apoptosis, differentiation, migration, and cell proliferation. It has been suggested that miRNA can be used for CRC diagnosis. Increased expression of miR-373 in serum and biopsies has been associated with CRC presenting acute symptoms and advanced stages during investigations. As a useful non-invasive biomarker, miR-373 expression patterns in serum can be detected even in the early stages of malignant rectal cancer. MiRNAs are gaining interest as potential biomarkers for diagnosis, prognosis, and prediction. Consequently, miR-373 analysis may serve as an additional, less intrusive, and more affordable screening technique to complement existing methods.
MiRNAs play a major role in the development and spread of CRC, serving as indicators for early CRC detection, therapeutic components for CRC formation, and potential targets for further study. MiRNAs, particularly miRNA-373, have the ability to function as oncogenes or tumor suppressor genes.