1. Background
Assisted reproductive technologies (ART) are recognized as innovative tools for improving reproductive efficiency and accelerating genetic progress. One of the most widely used ART techniques is in vitro embryo production (IVEP) (1). The success of this process largely depends on the intrinsic quality of oocytes and the composition of in vitro culture (IVC) media. In recent decades, extensive efforts have been made to identify suitable culture media for oocyte maturation. These efforts include the development of new formulations and the enrichment of these media with various components such as follicular fluid, antioxidants, cytokines, growth factors, and hormones (2).
Oocyte maturation is one of the critical stages in the in vitro production of embryos and is considered a major challenge in this system (3, 4). This stage has significant effects on the efficiency of embryo production. In vitro conditions expose oocytes to various stress factors, including oxygen tension and physical manipulations, which can disrupt their growth and development (4). Therefore, researchers have extensively studied various aspects of oocyte maturation across different species (5).
Morphologically, oocyte maturation involves physiological changes in the nucleus and cytoplasm. The extrusion of the first polar body is considered an indicator of nuclear maturation, while the expansion of cumulus cells is regarded as a marker of cytoplasmic maturation. Under in vivo conditions, nuclear and cytoplasmic maturation occur simultaneously; however, in in vitro conditions, nuclear maturation typically precedes cytoplasmic maturation (6). Nuclear maturation refers to the transition of oocytes from the arrest of the first meiotic division at the germinal vesicle (GV) stage to the arrest of the second meiotic division at metaphase II (MII) (7). This process occurs concurrently with cytoplasmic maturation, which involves structural changes such as the distribution and redistribution of cortical granules, storage of proteins and RNA, development of calcium regulatory mechanisms, and migration of mitochondria to the peri-nuclear regions (8). These events are initiated by the binding of gonadotropins to their receptors and the activation of G-proteins, triggering a cascade of phosphorylation in cyclic AMP (cAMP)-dependent protein kinases (9).
Additionally, the supplementation of oocyte maturation media with gonadotropins has been proposed as a strategy to enhance the developmental competence of oocytes (10). Gonadotropins play a particularly important role in increasing the number of oocytes reaching the MII stage and, consequently, the overall yield of viable embryos. These hormones alter the metabolism of cumulus cells and induce the resumption of meiosis by disrupting the inhibitory state through gap junctions in oocytes (11). Gonadotropins such as equine chorionic gonadotropin (eCG) and human chorionic gonadotropin (hCG) are used as alternatives to follicle-stimulating hormone (FSH) in the in vitro maturation (IVM) of mammalian oocytes (4, 12). The eCG, a glycoprotein extracted from the serum of pregnant mares, is recognized as an effective substitute for FSH. Due to its longer half-life and FSH-like activity, this gonadotropin has been widely applied in reproductive studies and treatments (13). The addition of eCG to oocyte culture media can increase the rate of MII oocytes in ovine (6). Similarly, hCG, which primarily mimics luteinizing hormone (LH), can alter calcium distribution in the cytoplasm and improve glutamine metabolism in oocytes when added to the maturation medium (14). Both hCG and eCG have been effectively employed in the IVM of mammalian oocytes (8). Moreover, commercially available eCG is more cost-effective compared to FSH and LH (15). Mingoti et al. demonstrated that eCG can enhance the expression of hCG receptors in cumulus cells, thereby enabling the activation of intracellular signaling pathways by hCG. Additionally, eCG has the ability to bind to both FSH and LH receptors (14).
In mammals, flavonoids exhibit a variety of biological and pharmacological effects. Research has shown that quercetin can possess anti-inflammatory and antioxidant properties, which arise from its activities in neutralizing free radicals and chelating metals. This compound also has strong antioxidant effects, but it can simultaneously induce pro-oxidant effects. There is a relationship between the free radical scavenging activity and the anti-carcinogenic and anti-inflammatory properties of quercetin (16). As a potential antioxidant, quercetin may reduce cellular apoptosis. Quercetin has been successfully used as a stimulant in the maturation of oocytes and pre-implantation and embryo development in ovine, goats, cattle, pigs, and mice in culture media (17). This compound is also capable of preventing mitochondrial dysfunction, neutralizing free radicals by removing oxidation products, and stimulating antioxidant enzymes (18). It appears that improving embryo production efficiency may be achieved through the inhibition of various systems involved in the apoptosis of oocytes (19).
2. Objectives
The use of an appropriate concentration of antioxidants can create optimal conditions for producing high-quality embryos. Additionally, gonadotropins exhibit significant similarities in their effects on ovarian hormones in vitro. Therefore, the aim of this study is to explore the role of gonadotropic hormones (eCG and hCG) and quercetin, as an effective antioxidant, in enhancing the TCM-199 maturation medium. This optimized maturation medium is then compared with the commercially available BO-IVM medium. This study was conducted as a randomized trial and investigated the effects of different culture conditions on the maturation of ovine oocytes.
3. Methods
3.1. Location, Chemicals, and Media
All experiments were conducted in the Embryo Biotechnology Laboratory at the Iranian Research Organization for Science and Technology (IROST), maintaining a consistent temperature range of 27 - 31°C. Chemicals and culture media were sourced from Sigma-Aldrich (USA) and Gibco (USA), while plastic materials were obtained from Falcon (USA), unless specified otherwise. All stock solutions and media were prepared using sterile triple-distilled Milli-Q water and filtered through 0.22 µm membrane filters.
3.2. Oocyte Collection
Ovaries from ovine were obtained from a local slaughterhouse and transported to the laboratory within 3 hours in a phosphate-buffered saline solution containing gentamicin (50 µg/mL) at a temperature of 25 - 30°C. The ovaries were trimmed to remove surrounding adipose tissue and were subsequently washed five times with physiological saline. A surgical blade was used to slice the ovaries for oocyte retrieval. The follicular fluid and cumulus-oocyte complexes (COCs) were placed in a small petri dish containing an aspiration medium enriched with TCM-199, L-glutamine (2 mM), bovine serum albumin (BSA) (0.3%), and gentamicin sulfate (50 µg/mL). Under a stereomicroscope, COCs were collected and gently washed three times using a washing medium. This washing medium was composed of TCM-199, L-glutamine (2 mM), sodium pyruvate (0.81 mM), fetal bovine serum (FBS) (10%), and gentamicin sulfate (50 µg/mL).
3.3. In Vitro Maturation
Cumulus-oocyte complexes exhibiting three or more layers of cells and a homogeneous cytoplasm were selected and matured in groups of up to 15 in 100 µL droplets of BO-IVM, TCM+, and TCM maturation media. The BO-IVM medium is a commercial culture medium that was considered as a control in this experiment, while the TCM medium was TCM-199 supplemented with 10% FBS, 10% ovine follicular fluid, 5 mg/mL FSH, 1 mg/mL estradiol-17β, 0.81 mM sodium pyruvate, and 50 mg/mL gentamicin sulfate. The TCM+ medium included the components of the TCM medium along with the gonadotropins eCG (20 µg/mL) and hCG (5 µg/mL), as well as the antioxidant quercetin (15 µg/mL). The maturation droplets were covered with mineral oil and cultured for 24 hours at 38.5°C in an atmosphere of 20% O2 and 5% CO2.
3.4. In Vitro Fertilization
The cryopreserved semen from a ram was quickly thawed at 37°C and washed twice with 10 mL of IVF medium, which consists of Brackett and Oliphant medium containing 10 µg/mL heparin, 137.0 µg/mL sodium pyruvate, and 1.942 mg/mL caffeine sodium benzoate. The samples underwent centrifugation at 1000 rpm for 5 and 7 minutes at room temperature to wash them twice. A hemocytometer was used to determine the concentration of the spermatozoa, which was then adjusted to 1.0 × 107/mL through further dilution. A 50 µL aliquot of the sperm suspension was mixed with a 50 µL droplet of the IVF medium. The 15 matured COCs were then placed into a droplet of 100 µL IVF medium and incubated for 18 hours at 38.5°C in a humidified atmosphere containing 20% O2 and 5% CO2.
3.5. In Vitro Culture
After fertilization, the presumptive zygotes were carefully washed by pipetting to remove any remaining cumulus cells and spermatozoa. The presumptive zygotes were then cultured in the BO-IVC medium to ensure consistent conditions for all. The zygotes were cultured for 9 days in a humidified atmosphere of 5% CO2, 5% O2, and 90% N2 at a temperature of 38.5°C.
3.6. Statistical Analysis
Each experimental group was subjected to three replicates. For the analysis of quantitative data, SPSS statistical software (version 16) was employed. The results are presented as mean ± standard error of the mean. Statistical evaluation was conducted using the analysis of variance (ANOVA) method, and differences were deemed statistically significant when P < 0.05.
4. Results
4.1. Experiment 1: The Impact of Maturation Media on Primary Embryo Development and Their Comparison
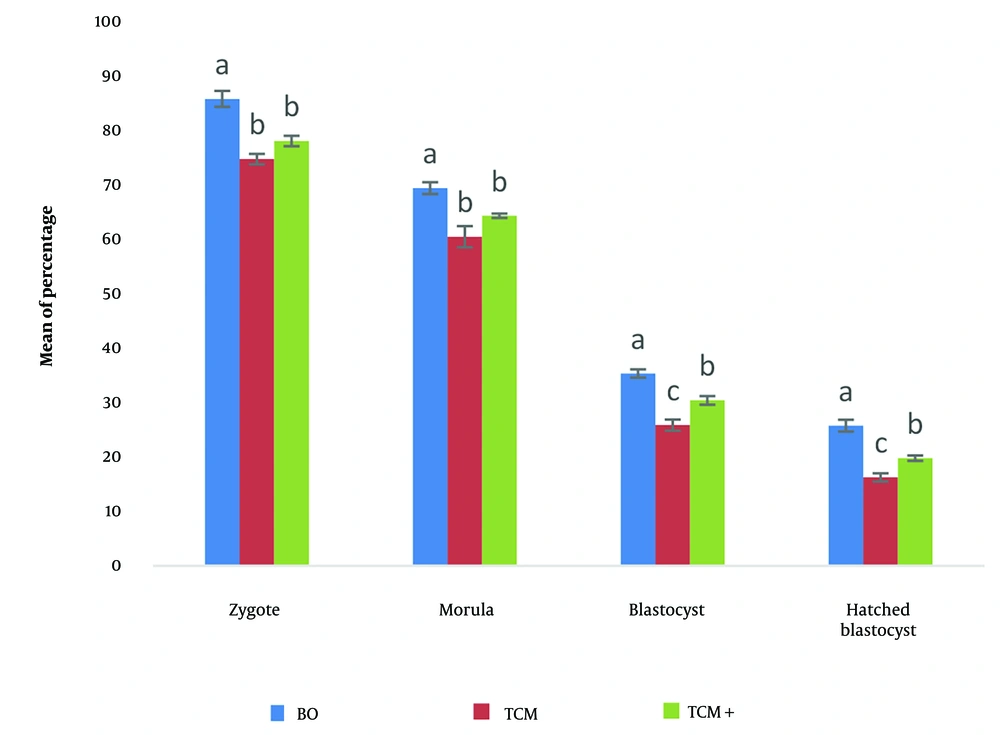
The mean percentage of zygotes, morulae, blastocysts, and hatched blastocysts in the BO-IVM medium was significantly higher compared to the TCM+ and TCM media (P < 0.05). Additionally, the mean percentage of zygotes and morulae in the TCM+ medium was numerically higher than in the TCM medium, but no significant difference was observed between these two media. The mean percentage of blastocysts and hatched blastocysts in the TCM+ medium was higher than in the TCM medium, with a significant difference observed (P < 0.05) (Figure 1).
4.2. Experiment 2: Examination of Gene Expression in Blastocysts Under Different Maturation Media
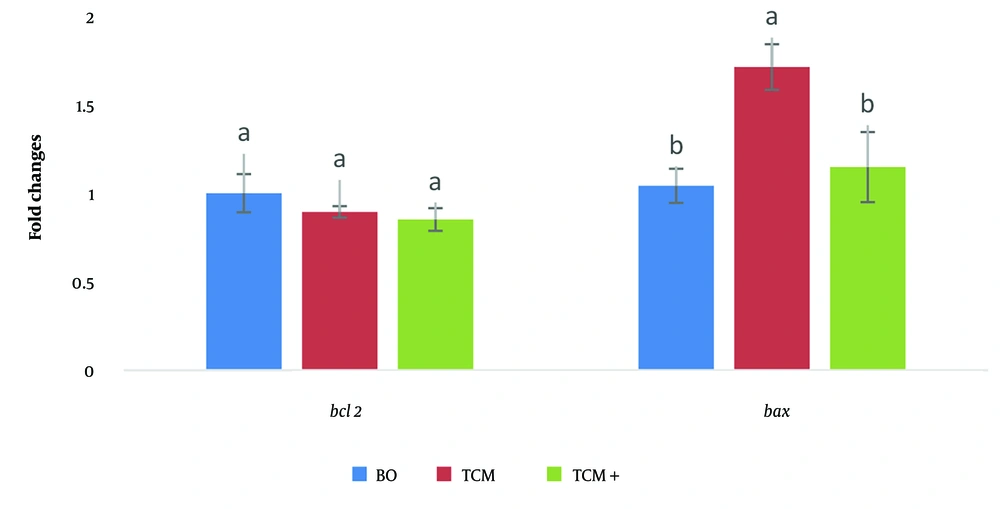
No significant difference was observed in the expression of the Bcl2 anti-apoptotic gene among the BO-IVM, TCM+, and TCM media. In the TCM medium, the expression of the Bax apoptotic gene was higher, with a significant difference noted compared to the other media (P < 0.05) (Figure 2).
5. Discussion
The results of this study revealed that supplementing the TCM medium with the antioxidant quercetin and the growth factors eCG and hCG not only improved blastocyst formation and hatching rates but also reduced the expression of the apoptotic gene Bax compared to the standard TCM medium. Gonadotropins, including eCG and hCG, are essential for oocyte maturation, influencing ovarian activity and promoting the development of viable oocytes. The eCG, which mimics FSH and LH, binds to FSH receptors and supports nuclear maturation, cumulus cell expansion, and mitochondrial activity, making it a suitable substitute for FSH in IVEP systems (8, 20). Human chorionic gonadotropin, similar to LH, binds to LHCGR in granulosa cells and is used as an LH substitute in vitro due to LH's rapid degradation (4). Studies have shown that eCG enhances cumulus cell expansion in species such as porcine, buffalo, and canine oocytes, though its effect on nuclear maturation varies (5, 6, 15). For example, Farag et al. (21) found that 10 µg/mL eCG significantly increases camel oocyte maturation rates, while the addition of 20 IU/mL eCG and hCG improves ovine oocyte maturation by enhancing cumulus expansion, polar body extrusion, and mitochondrial content (15). The inclusion of gonadotropins like eCG and FSH in maturation media boosts embryo development rates in buffalo and other species (20, 22). High concentrations of these hormones improve fertilization and embryo development, as demonstrated by Mogas et al. (23). Leisinger et al. (24) highlighted the synergistic effect of FSH and eCG on alpaca oocyte maturation, though lower concentrations reduce efficacy. Recent studies confirm that eCG enhances oocyte quality, mitochondrial activity, and gonadotropin receptor expression while reducing apoptosis (6, 8, 15). These hormones optimize nuclear and cytoplasmic maturation by increasing cAMP levels and improving cumulus-oocyte communication (20, 25). Overall, eCG and hCG play pivotal roles in improving oocyte maturation and embryo development across various species.
Kang et al. (26) reported the positive effect of quercetin on porcine oocytes, which improved embryonic development, reduced ROS production, and increased intracellular GSH levels at low concentrations; however, high concentrations were found to be detrimental. In another study, Kang et al. (16) attributed the decreased maturation rates of oocytes and blastocyst formation to the unresponsiveness of oocytes and embryos or toxicity from high levels of flavonoids. Research by Yu et al. (27) showed that quercetin enhances the quality of mouse embryos under oxidative stress conditions, leading to increased blastocyst formation and reduced apoptosis. Additionally, experiments on ovine oocytes demonstrated significantly higher cleavage and blastocyst rates, with quercetin reducing ROS accumulation and increasing overall antioxidant activity (28). Quercetin can also delay the onset of apoptosis and improve the quality of mouse oocytes (29). In goat oocytes, supplementation with quercetin resulted in decreased DNA fragmentation and increased percentages of oocytes at the MII stage (30). Studies have shown that ROS levels in quercetin-treated oocytes significantly decreased, while high doses performed poorly in reducing ROS levels (31). This compound reduces ROS levels in bovine oocytes and embryos by regulating the Nrf2-dependent oxidative stress response. Its beneficial effects on the antioxidant system and its role in reducing apoptosis in granulosa cells have also been documented (19). In their study, Davoodian et al. (19) reported that quercetin protects intestinal epithelial and endothelial cells from stress and apoptosis by modulating the expression of Bcl2 genes. Furthermore, treatment with quercetin in cumulus cells significantly decreased the expression of the Bax transcript, contributing to the oocyte's defense mechanism against apoptosis. Finally, quercetin protects human granulosa cells from oxidative stress and reduces endoplasmic reticulum stress-induced apoptosis in buffalo GCs, enhancing the ovarian antioxidant capacity by increasing the expression of certain oxidative stress-related genes in mice (32-34).
The results of this study indicated that although supplementing the TCM medium with the antioxidant quercetin and growth factors eCG and hCG improved its performance, the fertilization rate, morula formation, blastocyst formation, and hatched blastocyst rates were significantly lower compared to the commercial BO-IVM medium. The BO-IVM medium has been widely used in many studies (35-37) for embryo production. The BO-IVM medium includes essential components that enhance the maturation of oocytes in farm animals (38-41). Consistent with our results, Pryor et al. reported that specifically, embryos cultured in BO-IVC exhibited higher blastocyst rates and superior embryo quality.
5.1. Conclusions
The results of this study showed that the addition of the antioxidant quercetin and the growth factors eCG and hCG improved the performance of the TCM maturation medium by significantly increasing the average percentage of blastocysts and hatched blastocysts. However, the rates of fertilization, morula formation, blastocyst formation, and hatched blastocysts were significantly lower than those observed with the commercial BO-IVM medium. Therefore, further research is needed to enhance the performance of the TCM maturation medium in comparison to the commercial BO-IVM medium.