1. Background
Tendons play a crucial role in musculoskeletal function, serving as the connective tissue that transmits force from muscle to bone, thereby enabling movement and stability (1). They are subjected to mechanical stress and strain during daily activities and physical exercise, which makes them susceptible to injury (2). The healing process of tendons is complex and involves a cascade of biochemical and cellular events, including the recruitment of growth factors and the expression of genes associated with tissue repair (3). However, tendons exhibit prolonged healing processes due to their limited blood supply and slow cellular turnover. Tendon injuries — whether caused by overuse, trauma, or degenerative conditions — are a common challenge in both clinical and athletic settings, often resulting in significant functional impairments and a reduced quality of life (4).
Recent research has focused on identifying therapeutic strategies to enhance tendon healing. Among these, pharmacological interventions and physical exercise have shown promising potential (5, 6). Aerobic exercise, in particular, has been shown to improve blood flow, enhance metabolic activity, and stimulate the secretion of growth factors that facilitate tissue repair (7, 8). However, the underlying mechanisms by which aerobic exercise influences tendon regeneration remain largely unexplored. Meanwhile, bioactive compounds such as celastrol — a triterpenoid derived from the plant thunder god vine (Tripterygium wilfordii) — have gained attention for their anti-inflammatory, antioxidant, and pro-angiogenic properties. Celastrol has been shown to modulate key molecular pathways involved in tissue regeneration, making it an intriguing candidate for tendon repair therapy (9-11). While previous studies have demonstrated the benefits of celastrol in inflammatory conditions, its specific effects on tendon regeneration, when combined with aerobic exercise, remain unclear.
This study aims to investigate the effects of celastrol treatment alongside four weeks of aerobic exercise on Achilles tendon regeneration in male Wistar rats. The biological mechanisms underlying tendon regeneration involve a complex interplay of growth factors, cytokines, and extracellular matrix (ECM) remodeling. Accordingly, we assessed the synergistic effects of celastrol administration combined with four weeks of aerobic exercise on the local secretion of growth factors and the expression of genes associated with Achilles tendon regeneration.
2. Objectives
By exploring the interplay between celastrol treatment and aerobic exercise, this study endeavors to uncover novel therapeutic approaches that could accelerate tendon healing and improve functional outcomes. The findings have the potential to inform clinical practices and rehabilitation strategies, ultimately contributing to improved management of tendon injuries across diverse populations.
3. Methods
3.1. Animals and Experimental Groups
All experimental procedures adhered to ethical regulations for animal welfare and were approved by the Animal Use and Care Committee of Islamic Azad University. Male Wistar rats (220 - 250 g, 2 months old) were housed under controlled conditions with a 12-hour light-dark cycle, a maintained temperature of 22 - 24°C, and 60% humidity, with unlimited access to standard chow and water. The rats were randomly assigned to six groups (n = 16): Healthy control (no injury), negative control (Achilles tendon rupture, no treatment), sham (surgery without injury), experimental group 1 (injury + 4 weeks of training), experimental group 2 (injury + daily intraperitoneal injection of 2 mg/kg celastrol for one week), and experimental group 3 (injury + 4 weeks of training + 2 mg/kg celastrol administration for one week).
3.2. Surgery
To model Achilles tendon rupture, rats were anesthetized with an intraperitoneal injection of 80 mg/kg ketamine and 10 mg/kg xylazine (Alfasan). One cm lateral incision was made to expose the Achilles tendon, which was then completely transected 5 mm above the calcaneal insertion. Using a modified Kessler technique, the tendon ends were sutured with Ethicon 5 - 0 PDS Plus Antibacterial Polydioxanone Monofilament sutures (USA). After the skin was closed with 4 - 0 silk sutures, the rats were allowed to move freely and were monitored daily for signs of infection and wound complications.
3.3. Aerobic Exercise
The protocol for aerobic exercise on a treadmill for rats involved conditioning them with a sound stimulus and mild electric shock to initiate movement. Once conditioned, the sound stimulus alone was sufficient to prompt exercise. Rats that stopped more than five times within the first 10 minutes of exercise were excluded from the study. After a 3-day recovery period following surgery, the rats underwent four weeks of exercise, five days per week, with each session lasting 20 minutes. Treadmill speed began at 5 m/min, increased to 10 m/min during weeks 2 and 3, and reached 15 m/min in week 4. The rats were monitored for signs of fatigue and distress, and following each session, they were given access to rest, food, and water.
3.4. Enzyme-Linked Immunosorbent Assay
Four weeks post-surgery, tissue levels of fibroblast growth factor 2 (FGF-2), insulin-like growth factor 1 (IGF-1), and cyclooxygenase 2 (COX2) were measured in four animals from each group using ELISA. Tendon tissues were homogenized in Tris buffer containing sodium vanadate and protease inhibitors, then centrifuged. Protein concentration was assessed using the Bradford method. Subsequently, the concentrations of the target proteins were determined using the Rat FGF-2 ELISA Kit (R&D Systems, MFB00), the Rat IGF-1 ELISA Kit (R&D Systems, MG100), and the Rat COX2 ELISA Kit (Mybiosource, MBS266603), following the manufacturers’ instructions. Optical density at 450 nm was measured to quantify protein levels based on a standard curve.
3.5. Gene Expression Assay
Real-time polymerase chain reaction (RT-PCR) was conducted to estimate the mRNA expression levels of scleraxis, collagen type I, and matrix metalloproteinases (MMPs) in rat Achilles tendon tissue. Initially, total RNA was extracted from the tendon homogenate using a Total RNA Extraction Kit (Promega). RNA quality and quantity were assessed using a Thermo Fisher Nanodrop 2000 spectrophotometer. Subsequently, complementary DNA (cDNA) was synthesized from the extracted RNA using a Promega reverse transcription kit (CAT# A3500). The RT-PCR was then performed using specific forward and reverse primers for each target gene, along with nuclease-free water (ddH₂O) and Power SYBR Green Master Mix (2X, Invitrogen), to quantify gene expression levels on a Corbett thermal cycler. Amplification cycles were monitored in real time, and data analysis was conducted using the
| Symbols | Sequences 5'-3' | Product Size | Exon Junction |
|---|---|---|---|
| SCX | 195 | Yes | |
| Rat-SCX-F | GAACACCCAGCCCAAACAG | ||
| Rat-SCX-R | TGTGCTCAGATCAGGTCCAA | ||
| MMP3 | 162 | Yes | |
| Rat-MMP3-F | GAATCCCCTGATGTCCTCGT | ||
| Rat-MMP3-R | GGTCCTGAGAGATTTTCGCC | ||
| MMP9 | 139 | Yes | |
| Rat-MMP9-F | TGCCCTGGAACTCACACAA | ||
| Rat-MMP9-R | TGCAGGAGGTCATAGGTCAC | ||
| COLI | 280 | YES | |
| Rat-COLIA1-F | ATGAGTACCTGCTGGGAGG | ||
| Rat-COLIA1-R | CTAGGTCCTGCTGCTGCTG | ||
| GAPDH | 800 | YES | |
| Rat-GAPDH-F | TGCACCACCAACTGCTTAG | ||
| Rat-GAPDH-R | GGCATGGACTGTGGTCATG |
Characteristics of Primers Used in Real-time Polymerase Chain Reaction Studies
3.6. Histopathology
Briefly, Achilles tendons were harvested and fixed in 4% paraformaldehyde, then dehydrated through a graded ethanol series and embedded in paraffin. Sections (5 μm thick) were cut, deparaffinized, rehydrated, and stained with Masson's trichrome. The specimens were analyzed under a light microscope (YS 100, Nikon) for histological assessment.
3.7. Statistical Analysis
SPSS Statistics 20.0 (SPSS Inc., Chicago, Illinois, USA) was used for data analysis. The normality of data distribution was assessed using the Shapiro-Wilk test. One-way ANOVA was performed for multiple group comparisons, followed by Tukey’s post hoc analysis. Results were considered statistically significant at P < 0.05 and are presented as mean ± standard error of the mean (SEM).
4. Results
This study assessed the effects of a 4-week combination of training and celastrol on Achilles tendon rupture in rats. All animals survived without complications. Data from the healthy control group were omitted from the analysis due to the absence of significant differences compared to the sham-operated group, thereby simplifying the presentation of results.
4.1. Cytokine Levels
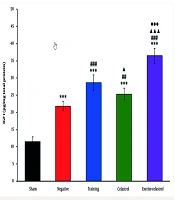
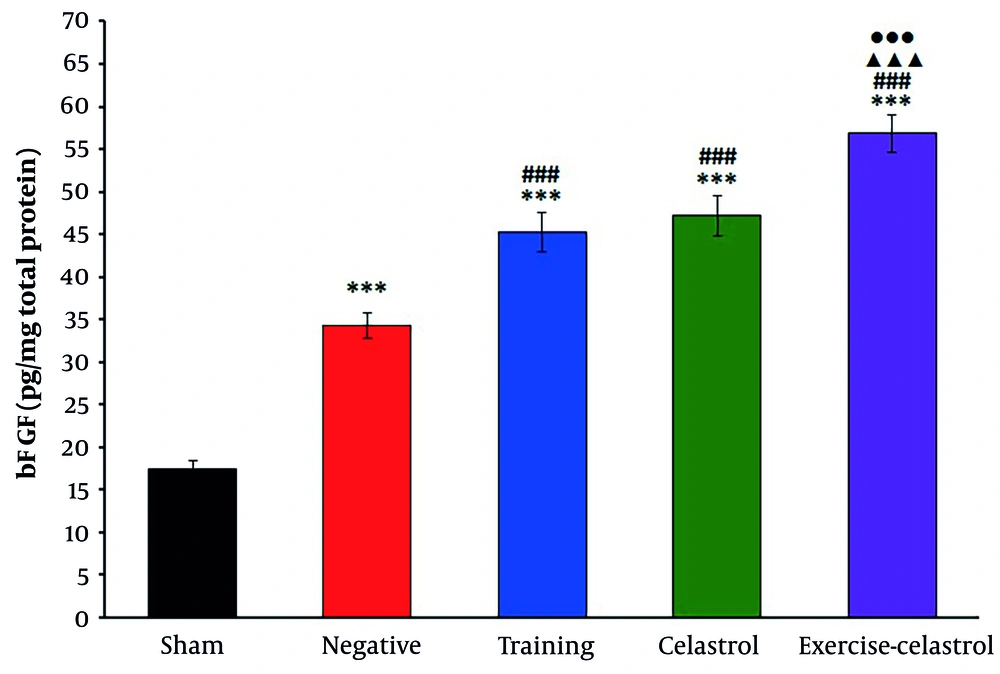
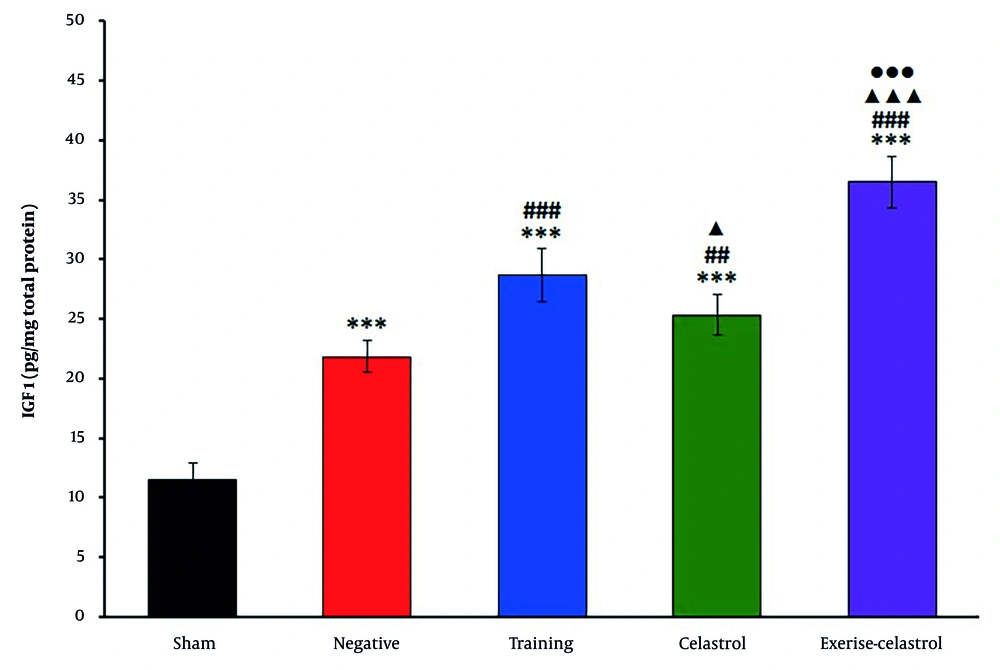
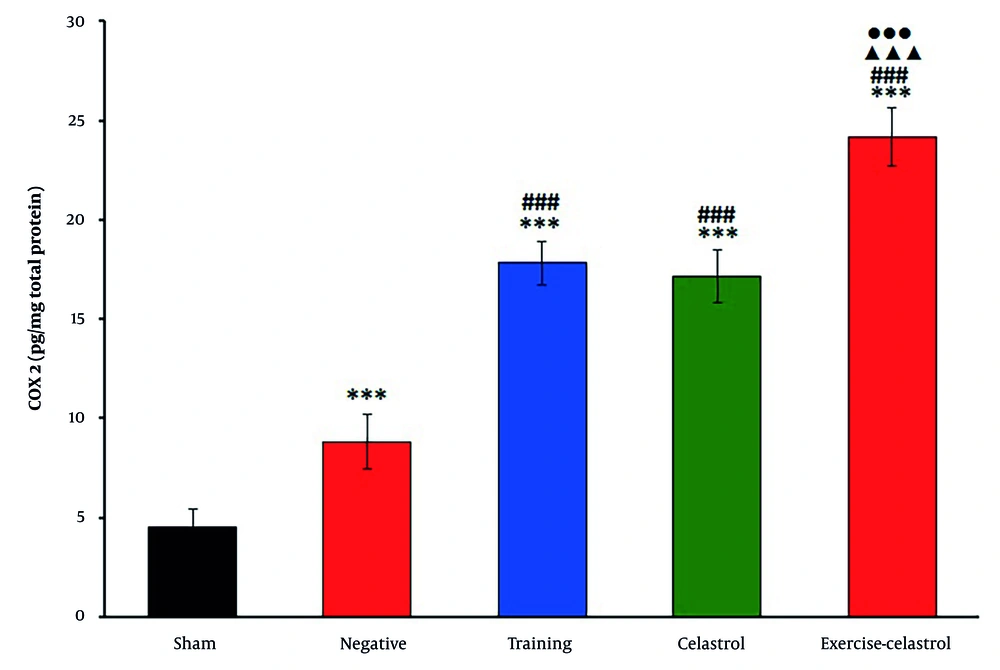
The ELISA results for bFGF expression in the tendons of healing rats revealed significant differences among the treatment groups (Figure 1). The sham group exhibited the lowest bFGF levels, while the negative control group showed a moderate increase. Both the training and celastrol groups demonstrated higher bFGF levels, with the training-celastrol group showing the highest expression, indicating a synergistic effect on tendon healing (P < 0.001). Similar trends were observed for IGF1 expression (Figure 2). The sham group again had the lowest levels, whereas the training and celastrol groups showed significant increases (P < 0.001). The training-celastrol group exhibited the highest IGF1 expression (P < 0.001), highlighting IGF1's pivotal role in tendon repair. The COX2 expression also varied significantly among the groups, with the sham group showing the lowest levels and the training and celastrol groups demonstrating elevated expression (Figure 3 P < 0.001). The highest COX2 expression was observed in the training-celastrol group (P < 0.001), emphasizing its critical role in tendon recovery. These findings underscore the effectiveness of the applied therapeutic strategies in enhancing tendon healing.
Enzyme-linked immunosorbent assay (ELISA) results for bFGF levels in the Achilles tendon of rats in experimental groups. Data were expressed as mean ± standard error (SE) (n = 4). *** P < 0.001 vs. sham group (untreated group); ### P < 0.001 vs. negative control group (untreated group); ▲▲▲ P < 0.001 vs. training group; ●●● P < 0.001 vs. celastrol group.
Enzyme-linked immunosorbent assay (ELISA) results for insulin-like growth factor 1 (1IGF-1) levels in the Achilles tendon of rats in experimental groups. Data were expressed as mean ± standard error (SE) (n = 4). *** P < 0.001 vs. sham group (untreated group); ## P < 0.01 and ### P < 0.001 vs. negative control group (untreated group); ▲ P < 0.001 and ▲▲▲ P < 0.001 vs. training group; ●●● P < 0.001 vs. celastrol group.
Enzyme-linked immunosorbent assay (ELISA) results for cyclooxygenase 2 (COX2) levels in the Achilles tendon of rats in experimental groups. Data were expressed as mean ± standard error (SE) (n = 4). *** P < 0.001 vs. sham group (untreated group); ### P < 0.001 vs. negative control group (untreated group); ▲▲▲ P < 0.001 vs. training group; ●●● P < 0.001 vs. celastrol group.
4.2. Real-time Polymerase Chain Reaction
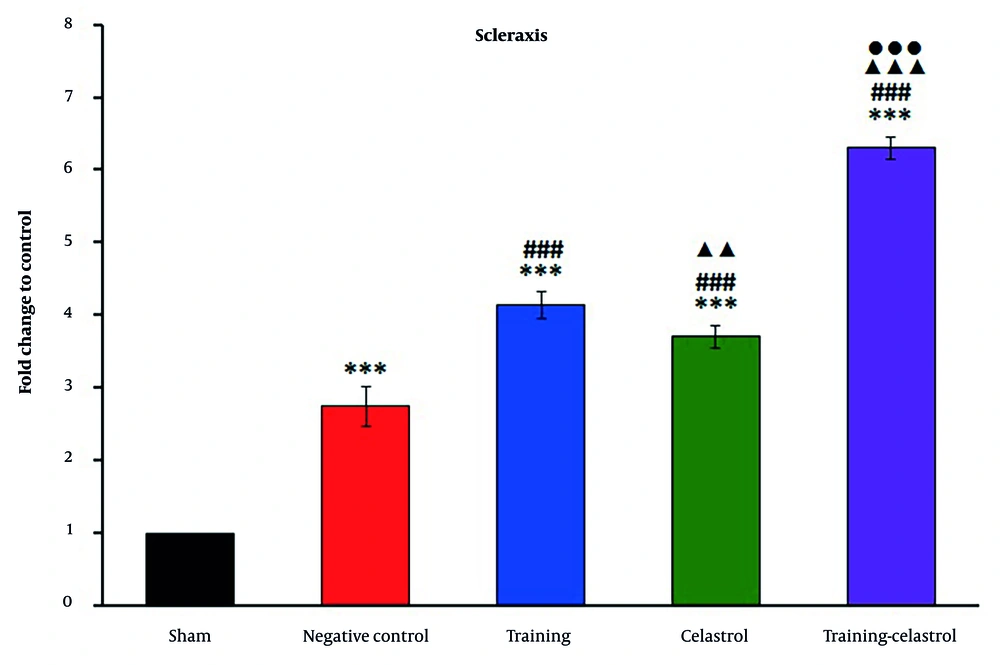
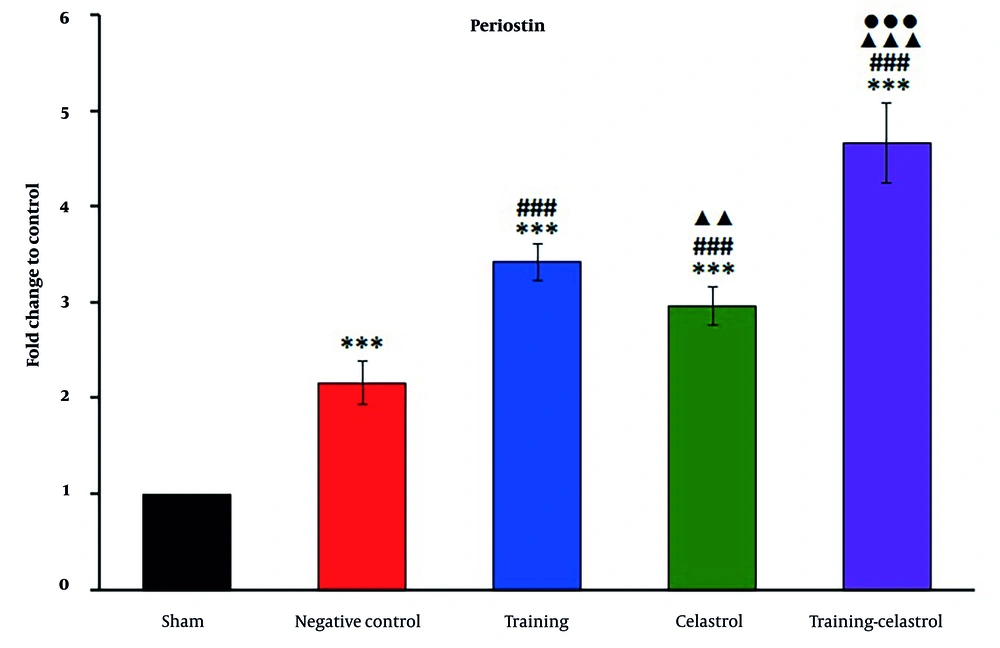
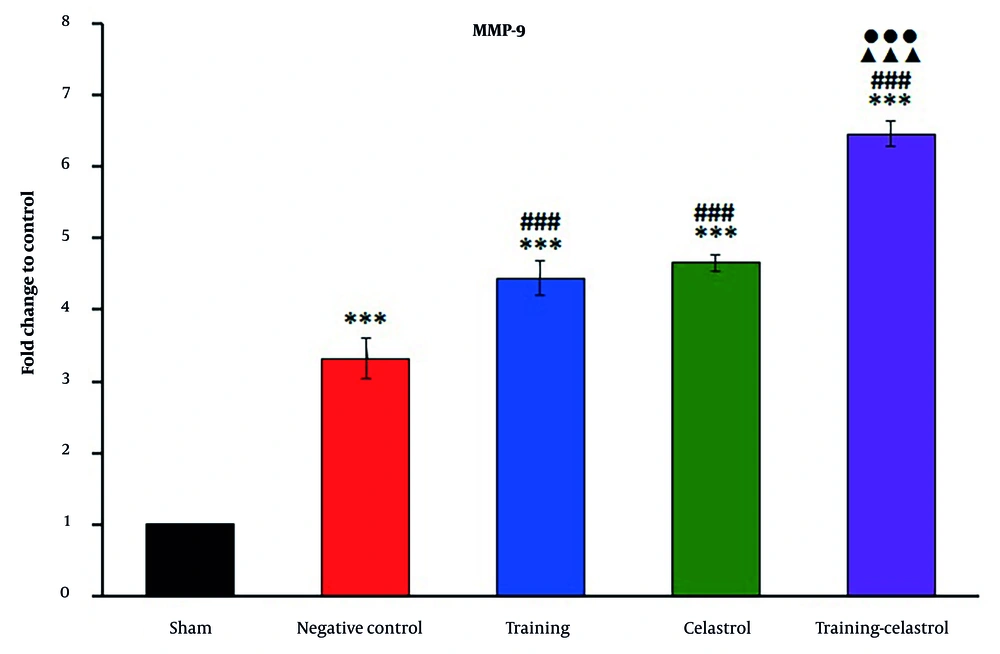
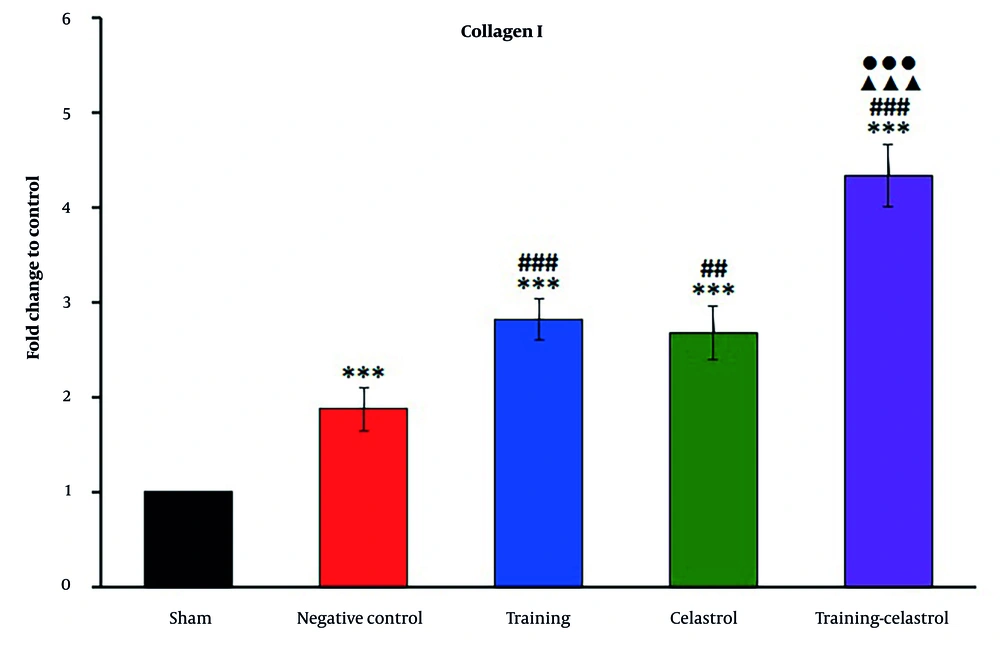
Real-time PCR analysis of the gene scleraxis in the tendons of healing rats revealed significant variations in expression levels across the treatment groups (Figure 4). The sham and negative control groups exhibited lower scleraxis expression, whereas the training and celastrol groups showed increased levels. The training-celastrol group demonstrated the highest expression (P < 0.001), indicating a synergistic effect on tendon repair. Similarly, the expression of periostin differed notably among the groups, with lower levels observed in the sham and negative control groups, and significant increases in the training, celastrol, and training-celastrol groups (Figure 5) (P < 0.001), highlighting their positive impact on tendon healing. For the gene MMP9, the sham and negative control groups also showed low expression (Figure 6), while the training and celastrol groups displayed marked increases. Once again, the highest expression was observed in the training-celastrol group (P < 0.001), emphasizing its role in tendon remodeling. Collagen I expression followed a similar pattern: Lower in the sham and negative control groups, but significantly increased in the training and celastrol groups (Figure 7). The training-celastrol group exhibited the highest expression levels (P < 0.001), indicating its vital role in tendon repair. Overall, these findings underscore the effectiveness of the applied treatments and the critical involvement of scleraxis, periostin, MMP9, and collagen I in the molecular mechanisms of tendon healing.
Real-time polymerase chain reaction results for scleraxis expression in the Achilles tendon of rats in experimental groups. Data were expressed as mean ± standard error (SE) (n = 4). *** P < 0.001 vs. sham group (untreated group); ### P < 0.001 vs. negative control group (untreated group); ▲▲ P < 0.01 and ▲▲▲ P < 0.001 vs. training group; ●●● P < 0.001 vs. celastrol group.
Real-time polymerase chain reaction results for periostin expression in the Achilles tendon of rats in experimental groups. Data were expressed as mean ± standard error (SE) (n = 4). *** P < 0.001 vs. sham group (untreated group); ### P < 0.001 vs. negative control group (untreated group); ▲▲ P < 0.01 and ▲▲▲ P < 0.001 vs. training group; ●●● P < 0.001 vs. celastrol group.
Real-time polymerase chain reaction results for MMP9 expression in the Achilles tendon of rats in experimental groups. Data were expressed as mean ± standard error (SE) (n = 4). *** P < 0.001 vs. sham group (untreated group); ### P < 0.001 vs. negative control group (untreated group); ▲▲▲ P < 0.001 vs. training group; ●●● P < 0.001 vs. celastrol group.
Real-time polymerase chain reaction results for collagen I expression in the Achilles tendon of rats in experimental groups. Data were expressed as mean ± standard error (SE) (n = 4). *** P < 0.001 vs. sham group (untreated group); ## P < 0.01 and ### P < 0.001 vs. negative control group (untreated group); ▲▲▲ P < 0.001 vs. training group; ●●● P < 0.001 vs. celastrol group.
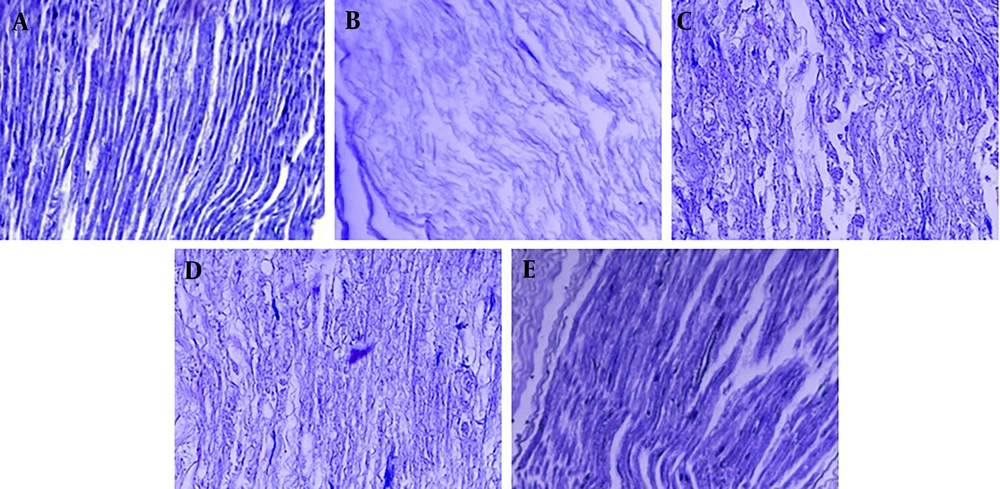
4.3. Histology
Based on the histological analysis using Masson's trichrome staining (Figure 8) conducted at the end of the fourth week, the sham group exhibited regular collagen organization. In contrast, the negative control group displayed disorganized collagen fibers, indicating incomplete healing. The training group showed improved alignment and deposition of collagen, while the celastrol group demonstrated enhanced collagen maturation and alignment, supporting previous observations. Notably, the training-celastrol combination group exhibited the most pronounced collagen fiber organization. These results reinforce the conclusion that the combined therapies of aerobic exercise and celastrol significantly promote tendon repair, enhance collagen alignment, and improve structural integrity.
5. Discussion
According to our results, the training-celastrol group exhibited the highest levels of bFGF and IGF1 — both critical growth factors for tendon healing — while the sham group had the lowest levels. Similarly, COX2 expression was highest in the training-celastrol group, underscoring its importance in the recovery process. The PCR analysis revealed that scleraxis, periostin, MMP9, and collagen I expressions were significantly elevated in the training and celastrol groups, with the combined treatment group showing the most pronounced expression, indicating a synergistic effect on tendon repair. Histological assessments corroborated these molecular findings, with the training-celastrol group demonstrating the most organized collagen fibers, reflecting enhanced structural integrity of the healing tendon. Overall, these results suggest that combining aerobic exercise with celastrol significantly enhances tendon healing by promoting the expression of key cytokines and genes involved in tissue repair and remodeling. Furthermore, the correlation between the ELISA and PCR data highlights how increased expression of bFGF, IGF1, and COX2 corresponds with higher levels of scleraxis, periostin, MMP9, and collagen I — all of which are essential for effective tendon recovery. In the context of tendon repair, the temporal dynamics of inflammation are critical, as successful healing requires a well-regulated inflammatory response. Early inflammatory signals, including the elevation of COX2, are necessary for recruiting immune cells and initiating tissue regeneration. However, prolonged or excessive inflammation can be detrimental to recovery. Our findings suggest that celastrol’s anti-inflammatory properties may effectively modulate this response, counterbalancing the pro-inflammatory effects of elevated COX2. This interplay highlights the importance of timing and regulation in inflammatory processes, indicating that a controlled inflammatory environment can enhance tendon healing outcomes. Future studies should further explore these dynamics to better understand the balance between inflammation and repair in tendon injuries. In addition, while our study appropriately emphasizes the role of scleraxis and collagen I in tendon repair, it is essential to recognize the dual role of MMP9 in ECM dynamics. MMP9 contributes to matrix degradation, facilitating the remodeling process necessary for effective healing, and plays a critical role in activating latent growth factors. This activation is crucial, as it enables the release of bioactive peptides that promote cellular proliferation and migration, thereby enhancing tissue repair. Thus, MMP9 serves as a key regulator in balancing matrix turnover with the availability of growth factors, underscoring its significance in the tendon healing process. Further investigation into this dual functionality could provide a more comprehensive understanding of MMP9’s contribution to tissue regeneration. Histological analysis further supports these findings, showing that enhanced cytokine and gene expression corresponds with improved collagen organization and alignment. This comprehensive approach underscores the importance of integrating exercise and pharmacological interventions to optimize tendon healing outcomes.
Aerobic exercises have been shown to significantly enhance the healing process of various tendon injuries. They increase blood flow and oxygen delivery to the affected areas, thereby promoting tendon cell activity and accelerating recovery (12). Furthermore, these exercises strengthen the muscles surrounding the tendon, helping to prevent overload and reduce the risk of re-injury. Aerobic activities also contribute to the regulation of inflammation and the balanced activity of tendinous cells, which supports a more effective healing process (13, 14). The gradual mechanical loading associated with aerobic exercises aids in the remodeling of tendon structure, enhancing its function and resilience (15). Numerous studies underscore the importance of aerobic exercise in tendon healing. For example, Fontana et al. investigated the combined effects of mesterolone and intensive treadmill training on Achilles tendon remodeling and observed significant structural improvements. Their findings suggest that mechanical stimuli from aerobic exercise and mesterolone can positively influence tenocyte morphology and tendon composition (14). Magnusson et al. reported that exercise enhances collagen expression and synthesis in tendons, thereby impacting their molecular structure and overall integrity (16). Alfredson et al. demonstrated the effectiveness of heavy-load eccentric exercises for treating chronic Achilles tendinosis, highlighting the therapeutic potential of targeted exercise protocols in promoting tendon healing (17). Cook and Purdam proposed a load-induced tendinopathy model, emphasizing the central role of mechanical loading and exercise in the management and treatment of tendon injuries (18). Kongsgaard et al. compared different therapeutic modalities — including eccentric decline squat training and heavy, slow resistance training — and found that both approaches improved tendon structure and function, further supporting the efficacy of exercise-based interventions (19). Additionally, Chen et al. showed that a progressive exercise rehabilitation program significantly improved healing outcomes in male mice with supraspinatus injuries by enhancing tissue fusion and tensile strength (20). Collectively, these studies affirm that aerobic exercise facilitates tendon healing by improving blood flow, enhancing muscle strength, modulating inflammation, and stimulating molecular and structural tendon remodeling.
Celastrol, a natural compound with anti-inflammatory and antioxidant properties, shows considerable promise in enhancing tendon healing through multiple mechanisms. It reduces inflammation in injured tendons, thereby creating a favorable environment for healing, while its antioxidant effects help mitigate oxidative stress, promoting healthier tissue recovery (21). Moreover, celastrol plays a regulatory role in MMPs, helping to maintain the balance between tissue degradation and synthesis (22). Several studies have demonstrated celastrol's role in tissue regeneration. Wu et al. found that celastrol enhances the differentiation and self-renewal capacity of tendon-derived stem cells (9). Wang et al. showed that celastrol alleviates joint pain and cartilage damage in osteoarthritis models by modulating gene expression pathways (23). Guan et al. reported that celastrol improved muscle health in diabetic rats by boosting antioxidant levels and enzymatic activity (24). Similarly, Gao et al. found that celastrol reduced inflammation and improved arthritis symptoms in rat models (21). Hu et al. also highlighted celastrol’s potential in promoting bone wound healing (10).
Despite significant research on tendon healing, the role of inflammation remains complex and not yet fully understood. Tendons typically heal through scarring, which often results in suboptimal functional outcomes. While inflammation is essential for tissue repair in regenerative contexts, its role in tendon healing is more problematic due to the tendon's limited regenerative capacity. Modifying the immune response during tendon injury may help foster a more pro-regenerative environment. Understanding the dynamics of immune cells and cytokines involved in tendon repair — particularly the timing and regulation of immune interventions — is critical to optimizing healing outcomes. Achieving a balanced inflammatory response is essential for effective tissue regeneration, as imbalances may lead to complications such as systemic inflammatory response syndrome or fibrosis. Emerging research highlights the involvement of a diverse range of cellular phenotypes in tendon healing, pointing to a complex interplay within the regenerative microenvironment (25). Additionally, factors such as age, sex, and underlying metabolic diseases must be carefully considered when examining inflammatory mechanisms in tendon regeneration. Continued advancements in our understanding of immune responses may pave the way for the development of immune-modulatory treatments aimed at enhancing tendon repair and improving clinical outcomes.
Conversely, the expression levels of scleraxis, collagen type I, and MMPs are essential in the healing and remodeling of Achilles tendon tissue. Scleraxis, a tendon-specific transcription factor, promotes tenocyte differentiation and stimulates collagen synthesis — particularly collagen type I, which is crucial for providing tensile strength (26). Elevated scleraxis expression enhances collagen production during tendon healing (27). Collagen type I is the predominant structural protein in tendons. Its expression is tightly regulated throughout the healing process, initially increasing during the proliferative phase and then transitioning into the remodeling phase to improve fiber alignment and mechanical strength (28). Maintaining a balance between collagen synthesis and degradation is vital; therefore, monitoring both collagen type I and matrix metalloproteinase (MMP) levels is essential (29). The MMPs, a group of proteolytic enzymes, facilitate ECM remodeling by degrading damaged collagen. However, excessive MMP activity can result in poor healing outcomes due to the breakdown of newly synthesized matrix components (30). Thus, a proper balance between MMPs and their natural inhibitors is critical for effective tendon repair. Research indicates that fluctuations in the expression of scleraxis, collagen type I, and MMPs significantly impact tendon healing (31, 32). Increased scleraxis expression correlates with enhanced collagen deposition, while dysregulated MMP activity may lead to excessive matrix degradation and compromised tendon structure. Understanding these molecular markers provides valuable insight into the pathophysiology of tendon injuries and offers direction for the development of targeted therapeutic interventions.
In contrasting our findings with those from chronic tendinopathy models, it is important to recognize the fundamental differences in the underlying pathophysiology between degenerative conditions and acute tendon ruptures. Chronic tendinopathy is characterized by a complex interplay of collagen disorganization, elevated MMP activity, and persistent inflammation, all of which impair healing and compromise structural integrity. In contrast, acute tendon ruptures typically follow a more straightforward healing trajectory, primarily focused on restoring the tendon’s structural integrity and function. Our study, which investigates the effects of aerobic exercise and celastrol on acute tendon healing, suggests a synergistic enhancement in healing outcomes — likely driven by increased collagen synthesis and improved fiber organization. In chronic tendinopathy models, however, therapeutic strategies may need to more directly target the underlying degenerative changes and persistent inflammation. This distinction underscores the necessity for tailored interventions based on the specific tendon pathology and highlights how our findings contribute to a broader understanding of tendon healing dynamics across varying injury types. Furthermore, it is critical to acknowledge species-specific differences in tendon healing. Rat tendons predominantly heal through scarring, whereas human tendons involve more complex processes, including regeneration and tissue remodeling. This disparity limits the direct applicability of findings from animal models to human clinical scenarios. While our results demonstrate promising effects of aerobic exercise and celastrol on tendon healing in rats, further research is essential to evaluate the translatability of these interventions to human tendon repair. Understanding these differences is vital for contextualizing our findings and guiding future studies aimed at developing targeted, clinically relevant strategies to enhance tendon healing outcomes.
5.1. Conclusions
This study examined the effects of combining aerobic exercise and celastrol treatment on Achilles tendon healing in male Wistar rats. The results indicated that this combined intervention significantly enhanced functional recovery, reduced inflammation, and modulated the expression of genes associated with tendon healing. Tensile testing and histological analysis further supported the therapeutic benefits of the combined treatment in promoting tendon regeneration. However, the study had several limitations, including a relatively small sample size and a short intervention duration of four weeks, which may not fully capture the long-term effects of the treatment. Therefore, future research involving larger sample sizes and extended follow-up periods is necessary to validate these findings and assess their translational potential for human tendon healing.