1. Background
Agricultural products are constantly exposed to pathogenic microorganisms, among which plant pathogenic fungi cause significant damage in vegetable fields worldwide (1). The damage to agricultural products caused by fungal diseases accounts for approximately 12% of global production, with a higher impact in developing countries (2). Most losses in agricultural products occur due to seedling death and plant death caused by the Phytophthora fungus (3). The genus Phytophthora, a major agent of plant death, has more than 70 plant hosts. This disease is prevalent in temperate and humid areas, especially in greenhouses. Chemical control of fungal pathogens and excessive use of fungicides in plant disease management lead to complications such as pollination disorders, cancer caused by toxic residues on food, environmental pollution, and the development of resistance against fungicides (4).
Using essential oils, plant extracts, and secondary metabolites is a novel method for controlling plant diseases (5). These substances contain complex compounds that act as toxic biological agents against pests and plant pathogenic fungi (6). Therefore, identifying and investigating these compounds can play an effective role in controlling pests and plant diseases (7, 8). Pathogenic fungi are the most significant cause of agricultural product losses in the field and post-harvest life, as well as increasing the mortality of honey bees. In general, the use of synthetic fungicides should be increasingly limited because, in addition to their negative impacts on the environment and human health, they have led to increased resistance in plant pathogens (9).
Recently, research into new applications of plant materials is increasing, as these materials contain many secondary metabolites that can be used to produce safe natural protective compounds (10). Various studies have highlighted herbal compounds with strong antifungal and antibacterial properties, such as the green shell of walnut (Juglans regia L.), which contains large amounts of polyphenols (11-13). According to researchers, phenolic compounds and flavonoids play a crucial role in plant defense mechanisms against pathogenic fungi (13). On the other hand, the use of some synthetic pesticides has been banned due to their high toxicity and long preharvest interval (PHI) periods (14). However, environmental pollution caused by excessive use and misuse of agricultural chemicals has led to significant changes in public attitudes towards pesticide use in agriculture. Biological control of plant diseases and pests is the best solution to mitigate the damages caused by synthetic pesticides (15).
The Indigo plant (Indigofera tinctoria L.), belonging to the Leguminosae family, originates from India. This medicinal plant is mentioned in traditional medicine for its benefits in promoting hair growth, treating epilepsy, chronic bronchitis, skin diseases like wounds, asthma, and cancer (16). Alkaloids, proteins, free amino acids, phenolic compounds, flavonoids, and other important compounds are found in I. tinctoria leaves. Many studies have noted the biological properties of Indigo, including antimicrobial, antioxidant, anti-seborrheic dermatitis, anti-psoriasis, anti-inflammatory, and anti-acne effects (17).
2. Objectives
The present review aims to provide comprehensive information on the antifungal effects of the Indigo plant under in vitro conditions.
3. Methods
3.1. Plant Sample Preparation
Indigo medicinal plant seeds will be cultivated in the medicinal plants collection of the Institute of Agricultural Research, Agriculture Institute Research, Institute of Zabol, Zabol, Iran. The aerial shoots will be collected during the flowering stage. After removing waste materials, the samples will be dried in the shade at normal room temperature and then crushed into small particles using an electric mill.
3.2. Extraction Methods
Ethanolic, methanolic, n-hexane, and acetone extracts will be prepared by cold maceration at a ratio of 1:10. Specifically, 10 g of plant powder will be soaked in 100 mL of the respective solvent in Zimax glass containers for 48 hours on a shaker at normal room temperature. The mixture will then be filtered through Whatman No. 1 filter paper.
3.3. Investigating the Effect of Plant Extracts on Pathogenic Fungi In Vitro
These experiments will be conducted at the Plant Biotechnology Research Institute, University of Zabol. To determine the growth rate and growth inhibition percentage (MGI), the following method will be employed. Initially, plant extracts will be mixed with PDA medium by dissolving each extract in 20% Tween and then combining them at concentrations of 60, 120, 180, 240, and 300 ppm. Ridomil-mancozeb powder, at a concentration of 1:000 in PDA medium at 40 to 45 degrees Celsius, will be used for fungicide treatment, while distilled water and 20% Tween will serve as the control culture medium.
After transferring the treatments to sterile Petri dishes and sealing them, 5 mm disks will be prepared from the edge of a 7-day-old fungal colony using a cork borer and transferred to the center of the aforementioned dishes. These will be incubated at a temperature of 25 degrees Celsius. The diameter of the fungal colony will be measured with a digital caliper on the 10th and 14th days, and the difference will be recorded as the colony growth rate. To determine the inhibition percentage of mycelium growth, the fungal growth rate will be measured 14 days after cultivation, and the inhibition percentage of growth will be calculated using the following formula (18). The LSD test will be used to compare data means.
A: Colony diameter in the control; B: Colony diameter in treatment.
4. Results
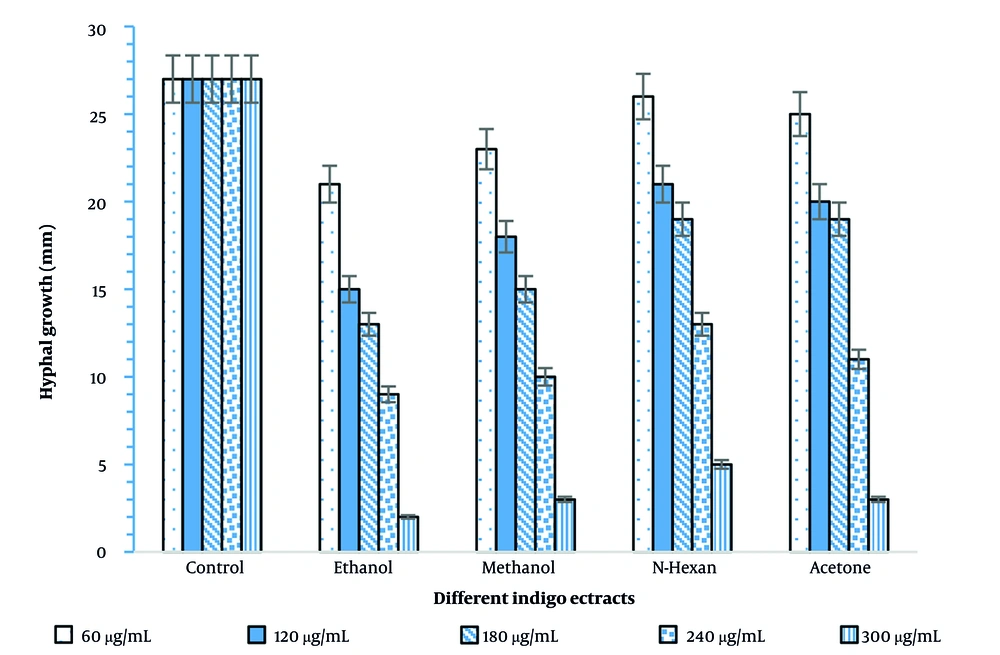
In vitro evaluation of the fungicidal effects of different extracts of the Indigo plant against Phytophthora drechsleri isolates demonstrated that the diameter of hyphal growth varied depending on the treatment and the duration of incubation. After 24 hours, the control treatment with distilled water was the least effective at all doses. The most significant growth inhibition was observed with the ethanolic extract at a concentration of 300 μg/mL, resulting in a hyphal growth diameter of 2 mm. In contrast, the diameter of colonies in the control was 27 mm, confirming a 92.5% inhibition of fungal growth (Figure 1).
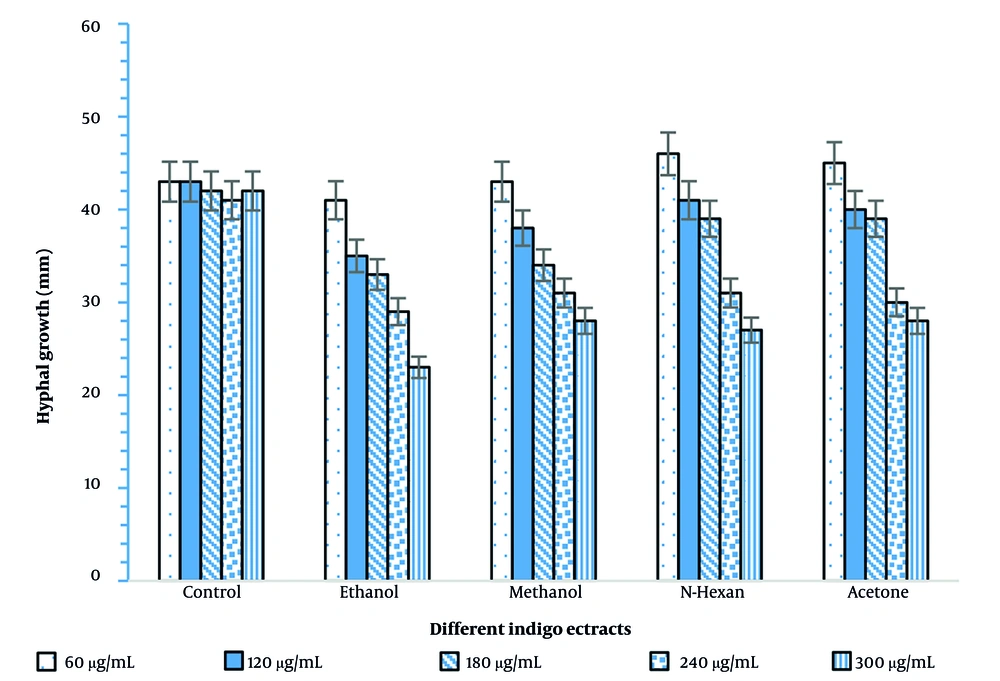
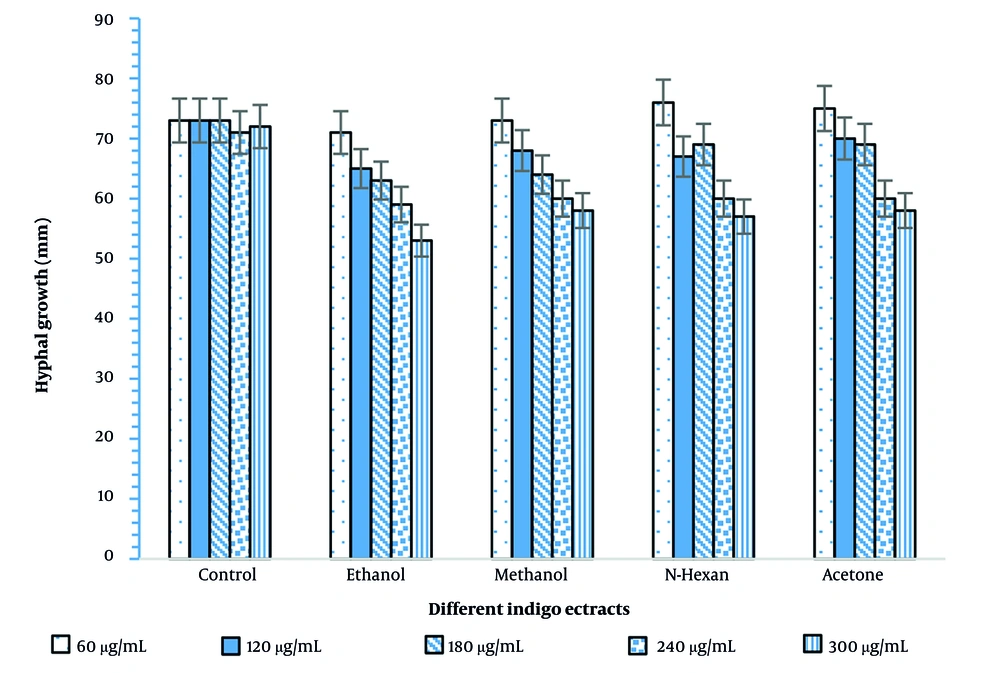
After 48 hours of incubation, the growth of Phytophthora drechsleri mycelium was clearly evident. In fact, the growth rate nearly doubled within 48 hours; however, by 73 hours, the mycelium exhibited a slower growth rate (Figures 2 and 3).
The results of the present study indicated that with increasing concentrations of Indigo extract, the inhibitory effect on the growth of fungal mycelium was enhanced. The highest inhibitory effect was observed at the 300 μg/mL concentration (Figure 3).
5. Discussion
The present results demonstrated that Phytophthora drechsleri affecting cucumber plants could be controlled by Indigo extracts in vitro. These findings suggest that ethanolic and methanolic extracts of the Indigo plant could effectively reduce the growth of Phytophthora drechsleri. Higher doses of these extracts, specifically at 240 and 300 μg/mL-1, were able to suppress the mycelium development of Phytophthora drechsleri and could be used to prevent infection by this fungus. These results align with those of Mehmet Colak (19), who reported the antifungal and antimicrobial properties of plant extracts from Indigo, demonstrating the inhibition of Rhodonia placenta and Trametesversicolor growth over 16 weeks according to the standard TS 5563-EN 113 (1996) method. Consistent with our findings, another study reported the antifungal activity of the butanol fraction of Indigo methanolic extract against Aspergillus niger, with a mean inhibition zone of 1.667 mm.
Other researchers have investigated the antibacterial, antifungal, and anthelmintic activities of Indigofera heterantha extracts. The results showed that at 100 mg/mL, methanolic bark extract inhibited Bacillus subtilis with a 20 mm zone of inhibition, followed by methanolic root with a 19 mm zone, while aqueous root, aqueous bark, and aqueous leaves showed no activity. Methanolic flower, aqueous root, and aqueous bark did not exhibit any antibacterial activity (20). Researchers have reported that Indigo is rich in bioactive compounds such as alkaloids, glycosides, tannins, flavonoids, and saponins, which are responsible for its medicinal properties (20).