1. Background
Nowadays, cancer is among the most important problems in medical science and patients with this disease are increasingly expanding. Cancer is a neoplastic disease that occurs due to abnormality in the cell cycle. Abnormality in the cell cycle and cell proliferation leads to uncontrolled cell proliferation and induction of cancer and tumor tissue. Gastric cancer is a major cause of cancer death (1). In terms of incidence, gastric cancer is the fourth most common cancer. It is also the second leading cause of cancer deaths around the world (2). Gastric cancer is a multifactorial disease that involves several environmental and genetic factors at different stages of the carcinogenic process. Studies have shown that genetic background, such as polymorphisms and mutations in genes involved in stomach cancer, play important roles in development of cancer (3, 4). One of the pathways for apoptosis is a mitochondria-mediated pathway that is controlled by members of the Bcl-2 family of proteins. The Bcl-2 family comprises of both anti-apoptotic and pro-apoptotic proteins. The anti-apoptotic members of this family, such as Bcl-2 and Bcl-XL (B-cell lymphoma-extra-large), prevent apoptosis by preventing the release of mitochondrial apoptogenic factors, such as cytochrome c in the cytoplasm. In contrast, pro-apoptotic members of this family, such as Bax and Bak (BCL-2 antagonist/killer), promote apoptosis by the secretion of cytochrome c from the mitochondria, which activate the apoptotic cascade and leads to cell death (5-7). Bax and Bcl-2 proteins are homologous in terms of structure. Bax protein is localized in the cytoplasm or cell membrane, yet Bcl-2 protein is localized in the nucleus and mitochondrial membranes (8-10). Indeed, the average proportion of these proteins determines cell fate (5). The Bax/Bcl-2 ratio determines the survival or death of the cell (7, 11). There are some chemical compounds that can change the Bax/Bcl-2 ratio and cause cancer cells destruction. A study on anticancer drugs showed that these drugs change the Bax/Bcl-2 ratio in the AGS cell line and cause apoptosis of the cancerous cell (12). Some compounds in plants can increase the Bax/Bcl-2 ratio and induce apoptosis of cancer cells. Pyrethrin is a herbal lethal substance for pests and its treatment on HepG2 cells (human liver cancer cell line) increases Bax/Bcl-2 ratio (13). Among the studied herbal derivatives and spices in relation to cancer, saffron (Crocus sativus L.) has received extensive attention. Saffron belongs to the Iridaceae family (14). It is said that saffron is a powerful antioxidant and anti-tumor agent (15). Saffron can be soothing for stomach pain, helpful for digestion, relieves renal colic pain, acts as an anti-depression, and increases appetite (16). The dried stigmas of saffron have therapeutic value due to three main secondary metabolites, including crocin (mono-glucosyl or di-glucosyl poly-n-ester) and its derivatives that are responsible for the red color of saffron, picrocrocin (mono terpene glycoside) that is responsible for the bitter taste of saffron, and safranal that is responsible for the smell of saffron (17). Many studies have been performed on the effects of saffron and its constituents in cancer prevention and treatment, yet the exact mechanism for these effects has not been identified. According to existing properties of saffron and its anti-tumor effect, and fewer side effects of medicinal plants than chemical drugs, this study investigated the anti-cancer effect of the extract of saffron on expression of Bcl-2 and Bax genes and expression rate of Bax gene compared to Bcl-2 gene (Bax/Bcl-2) in gastric cancer cell line (AGS), by real-time PCR method.
2. Objectives
The aim of this study was to investigate the anti-cancer properties of saffron extract and its effect on expression of Bcl-2 and Bax genes in gastric cancer cells (AGS) by real-time PCR.
3. Methods
3.1. Preparation of Saffron Extract
Saffron was supplied by Saharkhiz Co. (Mashhad, Iran) and was processed at the Biology Research Center of Islamic Azad University of Zanjan Branch, Iran. To prepare different concentrations of saffron extract, 5 g of saffron powder was dissolved in 200 mL of sterile distilled water. The obtained solution was held for 24 hours in a shaking incubator. After 24 hours, the solution was filtered twice by the filter paper. The solution volume reached 90 mL by a rotary device. Then the solution was sterilized by 0.22 micron filters and concentrations of 0, 800, 1200, and 2000 μg/mL were prepared (18).
3.2. Cell Culture and Treatment
In this study, the AGS cell line (human gastric adenocarcinoma, IBRC C10071, Iran) was purchased from Iran National Genetic and Biological Resources Center. In this experiment, a culture medium containing 89% DMEM (Dulbecco’s modified eagles medium), 10% FBS (fetal bovine serum) and 1% serum antibiotics was used. The DMEM 1X (Cat. No. 10-DM2-500) used in this experiment contained L-Glutamine, 4500 mg/L D-glucose, and pyruvate, purchased from the Inoclon company. FBS (Cat. No. 12-FB1-100), used in this experiment, was purchased from the Inoclon company. Serum antibiotics penicillin-streptomycin (pen/strep 10000 U/mL) with Cat. No. 15140-122 was also purchased from the Gibco company. It is important to notice that phosphate buffered saline (PBS) and trypsin-ethylene diamine tetra acetic acid (EDTA) were used in this project. Furthermore, PBS 1X used in this experiment was without calcium and magnesium with Cat. No. 11-PB1-500 and was purchased from the Inoclon company. Trypsin-EDTA 1X contains 2.5 g/L of trypsin and 0.38 g/L of EDTA. Trypsin-EDTA with Cat. No. 12-TR2-100 was also purchased from the Inoclon company.
Initially, AGS cells were cultured in medium containing DMEM, in a 37°C incubator, containing 5% carbon dioxide. The 1 × 105 AGS cells were cultured on 12 wells and cell status was evaluated before treatment. Cultured cells were divided to two groups. One group of cells were not treated (0 μg/mL), yet the other group were treated with different aqueous extracts of saffron (800, 1200 and 2000 μg/mL) at 48 and 72 hours.
3.3. Cytotoxicity Assay
The effect of saffron extract on AGS cells was determined in two groups during 48 and 72 hours. Its purpose was studying the cytotoxicity effect of saffron extract and survival of AGS cells. In this experiment, the cells were seeded in 96-well plate. After 24 hours, saffron extract was added to the AGS cells at concentrations of 200, 400, 800, 1200, and 2000 μg/mL. Cells were incubated in two groups for 48 and 72 hours. After that, 200 mL of the MTT solution (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) was added to the AGS cells and incubated for four hours. Then, 100 μL of the solution of DMSO (dimethyl sulfoxide) was added and optical density was measured at 570 nm by the ELISA Reader. In this experiment, the value of IC50 was calculated from saffron extract at specific doses and times. This experiment was performed in triplicates and the results were obtained as mean ± SD.
3.4. RNA Extraction and cDNA Synthesis
To collect the cells after treatment, the cells were washed twice with PBS solution and were then trypsinized. Cells were placed in 1.5-mL micro tubes and centrifuged at 1800 rpm for five minutes. Then 1 mL of PBS was added to the cells sediment and centrifuged at 3000 rpm for five minutes. RNA was extracted from AGS cell by Cinna pure RNA kit (Cat. No. PR891620) from Sinaclon company. The steps for extracting RNA were as follows:
1. Supernatant was completely removed by pipetting. Dislodged cells entered the plate by soft hitting, then 400 μL of lysis buffer was added. The cells were disrupted and homogenized by vortexing for one minute.
2. By adding 300 μL of precipitation solution, the lid was closed and inverted for ten times.
3. By transferring the solution to a spin column with collection tube by pipetting, the tube was centrifuged at 13000 rpm for one minute. After flow-through was discarded, 400 μL of wash buffer I was added to spin column and centrifuged at 13000 rpm for one minute.
4. The spin column with 400 μL of wash buffer II was washed and centrifuged at 13000 rpm for one minute. Flow-through was then discarded.
5. The solution was centrifuged at 13000 rpm for one minute and this step was repeated again.
6. The spin column was transferred to a new micro-centrifuge tube.
7. 50 μL of 55°C pre-heated RNase free water was placed in the center of the column, the lid was closed and incubated for four minute at 55°C and then centrifuged at 13000 rpm for one minute.
Furthermore, the concentration and purity of RNA were investigated by a spectrophotometer.
Next, 1 μL of RNA and 99 μL of sterilized water, distilled twice, was mixed and then 1 μL was poured in the cuvette and was placed in a spectrophotometer. The concentration of RNA and OD260 and the OD260/OD280 ratio were determined.
cDNA was the synthesized from extracted RNA. The Cycle Script RT PreMix (dN6) kit (Cat.No. K-2046-R) that was produced by the Takapozist company had been used for cDNA synthesis. One μL of RNA and 20 μL of DEPC (diethyl pyrocarbonate) water were added to RT-PCR kit tubes, and after spinning were placed in the thermal cycler. The PCR temperatures were as follows: Denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds and extension at 72°C for 30 seconds and 30 cycles.
3.5. Primers
The forward and reverse primer sequences for RT-PCR and Real-time PCR was selected according to previously published articles, as follows. The primers are shown in Table 1.
3.6. Real-time Polymerase Chain Reaction
The real-time PCR kit (AMPLIQON, Real Q Plus 2x Master Mix Green, High Rox, Cat.No. A325406) was used in this experiment from the Virgin company. Expression levels of Bcl-2 and Bax were evaluated by real-time PCR. The GAPDH gene was also considered as a reference gene to compare the expression of genes. At this stage, the researchers used a 0.2-mL micro-centrifuge tube for DNase and RNase Free. The constituent parts and its values for making the Master Mix were as follows:
To dilute 5 μL of cDNA for each group, 95 μL of sterile double distilled water was used. For each micro tube, 10 μL of diluted cDNA and 15 μL of Master Mix of each gene were added. Each kind of Master Mix of Bax, Bcl-2, and GAPDH genes include: 125 μL of SYBR Green, 5 μL of sterile double distilled water, 10 μL of F primer, and 10 μL of R primer.
To correct the probability of contamination during the real-time PCR process, the no template control (NTC) was used. From all of the NTCs, there were two NTCs that contained the following: The first one contained 15 μl of the F primer plus 10 μL of sterile double distilled water and the second one contained 15 μL of the R primer plus 10 μL of sterile double distilled water. Also, one NTC contained sterile double distilled water to 12.5 μL and a SYBR Green solution of 12.5 μL.
The real-time PCR reaction timing and temperature schedule was performed on the 3000 gene. Thermal cycler (Corbett-Rotor) device: First, a cycle was conducted to activate the Taq polymerase enzyme and to separate the two-stranded DNA of the primary pattern for 15 minutes at 95°C. The next step was alternately performed for 40 cycles during 15 seconds at 95°C and then for 30 seconds at 60°C, and then for 30 seconds at 72°C. To evaluate the function and characteristics of primers, the reactions were performed as duplicates.
In the end, it is necessary to draw a threshold line. The cycle that is interrupted by the threshold line is called cycle threshold (CT). Finally, the CT was calculated for each gene in its own group.
• ∆Ct (control) = Ct (target gene) - Ct (reference gene)
• ∆Ct (treatment) = Ct (target gene) - Ct (reference gene)
• ∆∆Ct = ∆Ct (treatment) - ∆Ct (control)
• Relative fold change = 2-∆∆Ct
t-test was used for significance and mean comparison of each group with the control group, and P < 0.05 was considered as significant.
3.7. Statistical Analysis
In this experiment, all stages were repeated three times. Thus, three times high-purity RNA extraction was performed and its cDNA was synthesized, then three times the reaction of the real-time PCR was performed. The results were calculated by using the 2-∆∆Ct method for two groups, during 48 and 72 hours. The expression of genes by method 2-∆∆Ct was obtained by the following formulas and the process of reducing or increasing genes was studied. Results were investigated as mean ± SD of the indicated number of independent experiments.
4. Results
4.1. Cytotoxicity of Saffron Extract
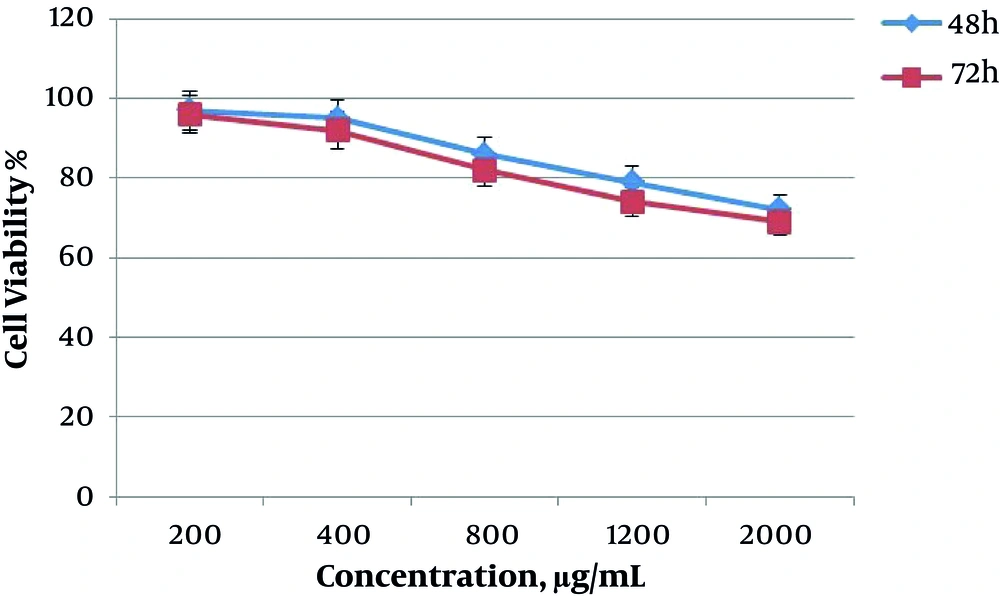
The results obtained from MTT assay (value of IC50) showed the cytotoxicity of saffron extract on the death of AGS cells. The inhibitory effect of saffron extract was dependent on time and concentration of extract and with the addition of time and concentration of the extract, the cytotoxicity of saffron was increased. In Figure 1, the graph shows the effect of saffron extract on the survival of AGS cells at doses 200, 400, 800, 1200, and 2000 μg/mL in two groups during 48 and 72 hours. The highest cytotoxicity was observed at 800, 1200 and 2000 μg/mL. Also, at 72 hours, the percentage of cell survival was lower.
4.2. Changes of Genes Expression
Expression of Bax and Bcl-2 genes and GAPDH gene as internal control were investigated in two groups of 48 and 72 hours with real-time PCR. Figure 2 shows an example of the replication of these genes by primers.
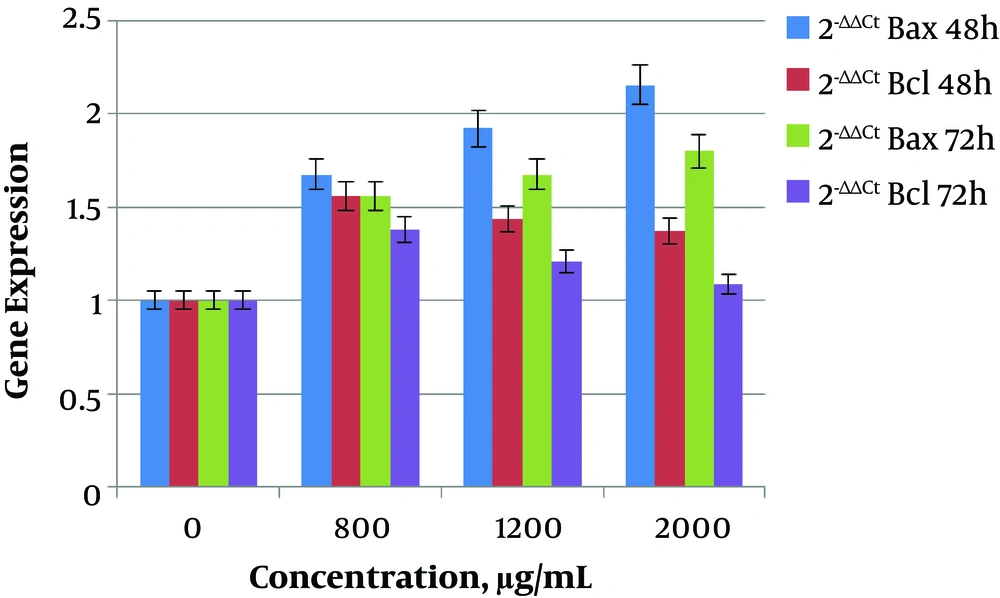
In this study, the expression of Bax gene showed a significant increase (P < 0.05) at 800 (0.000004987), 1200 (0.00004734), and 2000 μg/mL (0.0007998) doses of saffron extract in 48 hours in comparison to the dose of 0 μg/mL. The expression of Bax gene increased with increasing of dose concentration, and the highest expression was at 2000 μg/mL. The expression of Bcl-2 gene also showed a significant increase (P < 0.05) at 800 (0.00006787), 1200 (0.02131), and 2000 μg/mL (0.0195) doses in 48 hours in comparison to the dose of 0 μg/mL. By increasing the dose concentration, the expression of Bcl-2 gene decreased and the lowest expression occurred at 2000 μg/mL. On the other hand, at 72 hours the expression of Bax was increased by increasing of dose, and the expression of Bcl-2 was decreased by increasing the dose in comparison to the dose of 0 μg/mL.
The obtained results showed that the ratio of Bax/Bcl-2 at 48 and 72 hours was increased with increase of dose. In the group of 48 hours, the ratio of Bax/Bcl-2 increased significantly at concentrations of 800 (0.0405), 1200 (0.01935), and 2000 μg/mL (0.008017) of saffron extract. In the group of 72 hours, the ratio of Bax/Bcl-2 also increased significantly at concentrations of 800 (0.04018), 1200 (0.02088), and 2000 μg/mL (0.0007212).
It should be noted that at the dose of 2000 μg/mL, the highest ratio of Bax/Bcl-2 was obtained. Also, the ratio of Bax/Bcl-2 at 72 hours was more than 48 hours in all of the doses. The changes in the relative expression of the genes in the group without saffron extract and in groups of 800, 1200, and 2000 μg/mL are presented in Tables 2 and 3 in two groups of 48 and 72 hours. The columnar chart for changes in relative expression of Bax and Bcl-2 genes in two groups of 48 and 72 hours are shown in Figure 3.
aData are presented in mean ± SD.
aData are presented in mean ± SD.
5. Discussion
Bax gene is a key gene for apoptosis, which leads to the release of cytochrome C from mitochondria and activates the apoptotic cascade and it is the reason for cell death, while Bcl-2 gene prevents release of cytochrome C (7, 8). Increase in the expression ratio of Bax on Bcl-2 genes caused cancer cells to promote cell death (11). Many herbal drugs change the ratio of Bax/Bcl-2 and induce apoptosis in cancerous cells. Anti-cancer and cytotoxic properties of saffron have been reported in previous studies. Tavakkol-Afshari et al. studied the effect of saffron extract on HepG2 (hepatocellular carcinoma) and HeLa cell lines. They reported that saffron could decrease cell viability in malignant cells, in a concentration and time-dependent manner (18). In another study, Geromichalos et al. showed that crocin and safranal of saffron, have cytotoxic response and anti-tumor activity in colorectal cancer (21).
The cited examples prove that saffron is anti-cancerous in the development of cancer cells. In the present study, anti-cancer properties of saffron extract on cancer cells was demonstrated. The extract effects also changed the expression of the Bax gene. The changes of this expression were incremental and it has been seen in all cancer cell groups. The over expression of Bax was ascending with increasing of the extract concentration. With increasing of saffron extract dose, the expression of Bax gene also increased in both time periods. On the other hand, Bcl-2 gene expression increased in AGS cells at both time periods, yet with increasing of extract dose, the level of expression decreased.
Hoshyar et al. studied action mechanism of crocin in AGS cells and HFSF-PI (normal human fibroblast skin cells). They also compared the ratio expression of Bax/Bcl-2 before and after treatment with the saffron extract. They showed that crocin is the reason for the change in the ratio of the Bax/Bcl-2 expression, leading to apoptosis (11). In the another report, Yan Sun et al., studied the effect of crocin on human leukemia (HL-60) cell line and reported changes of expression between Bax/Bcl-2 genes. They showed that crocin prevents the proliferation of cancer cells, that occur by increasing ratio of Bax/Bcl-2, leading cells to apoptosis (22). Mousavi et al. investigated caspase proteins and Bax gene in MCF-7 cells (breast cancer cell line / Michigan Cancer Foundation-7) after treatment with saffron extract. They reported that saffron extract causes apoptosis of cancer cells and increases the expression of Bax in these cells (15). The investigated cells in the control group, showed that the expression of Bax was reduced compared with Bcl-2 in cancer cells. Treatment of gastric cancer cells with 800, 1200 and 2000 μg/mL of saffron extract for 48 and 72 hours, induced changes in the Bax and Bcl-2 genes expression. Increasing the dose and time, increased the ratio of expression of Bax on Bcl-2 (Bax/Bcl-2) and led to cancer cell death. Saffron properties can be used in the production of anti-cancer drugs and the results of this study can help treat cancer patients.