1. Background
Multiple sclerosis (MS) is neurodegenerative disease, which affects the central nervous system (CNS) (1), Furthermore, MS is an autoimmune disorder, which interferes with the immune system causing myelin sheath degradation. Symptoms of MS involves: vision, sensory and motor systems, balance and coordination, bowel/bladder/sexual, and cognition disorders (2, 3).
EDSS (expanded disability status scale) of patients was assumed from 0 to 10 based on the Kurtzke expanded disability status scale (4).
MS is clinically separated into four diverse categories: RRMS (relapsing-remitting), SPMS (secondary-progressive), PRMS (progressive-relapsing), and PPMS (primary-progressive) (5). A total of 85% of individuals who identified with MS have primary signs or attacks, which is mentioned to clinically isolated syndrome (CIS). Average onset of MS is between 20 and 40 years old (5). The MS incidence in childhood and after the age of 50 is rare (6). The peak of MS incidence is around the age of 24 (7). Women have two or three-fold more prevalence of multiple sclerosis than men (6).
Both environmental and genetic factors trigger MS (8). The first idea regarding genetic factors was raised in the 1890s (9, 10). In the 1920s, worldwide investigation of MS prevalence demonstrated a racial and ethnic difference, which may indicate a genetic influence (11).
Several genes contribute in MS susceptibility such as major histocompatibility complex genes (1).
Major histocompatibility genes, such as HLA, are integrated in normal immune response. The term HLA refers to the human leucocyte antigen system (12). Several of these genes play leading role in numerous immunological progressions (13).
Certain alleles of HLA have been studied in MS susceptibility. HLA-A3 (HLA-A*03) is one allele of class I HLA, which is firmly related with MS (1).
HLA-A*03 frequency in the Khuzestan province has not yet been reported. Ethnicity diversity in Khuzestan and limited published data for local Arabs and nearby located Arab countries is the turning point of our data.
Our goal was to consider the association between HLA-A*03 in MS patients and healthy controls from the Khuzestan province as well as study this allele in different ethnicities (Arab and Persians). Furthermore, we examined the possible relationship of HLA-A*03 allele with course of disease, primary symptoms, and EDSS.
2. Methods
A total of 200 MS patients, according to the McDonald criteria, confirmed in the Khuzestan multiple sclerosis society, were studied in Shahid Chamran University, (159 females and 41 males with the mean age as 32.2 years and age range 16 - 57). Clinical symptoms estimation was conduct by expert neurologist. Furthermore, we included 195 unrelated healthy controls excluded for any possible autoimmune-disease and history of MS in the family to this study (165 women and 30 men, mean age was 27.4, and age range was 14 - 63). The control group was selected from the same geographical zone and matched with patients on characteristics such as ethnicity, gender, and age. All contributors were informed regarding the aim of the study and consent form were completed by all.
A total of 5 mL of blood was drawn with the consent of patients and controls and collected in EDTA tube.
DNA extraction and purification was performed by high pure PCR template preparation kit from Roch Company, Germany.
2.1. Primers
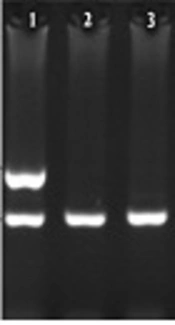
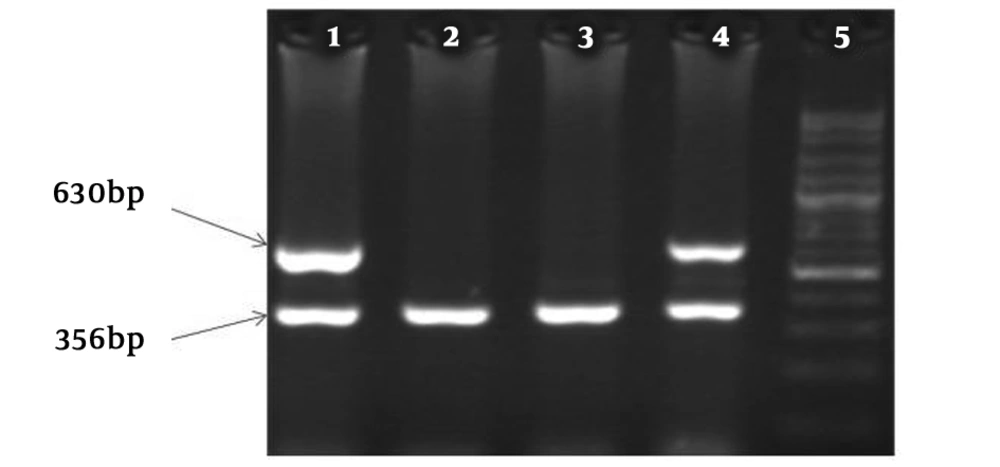
HLA-A*03 allele in MS patients and health controls was investigated by sequence specific primer polymerase chain reaction (SSP-PCR). Primers sequences was applied to amplify the HLA-A*03 designed according to IMGT/HLA database (http://www.ebi.ac.uk/) and checked out by NCBI/blast database (www.ncbi.nlm.nih.gov). Forward and reverse primers sequences for HLA-A*03 was: Forward: 5’-AGCGACGCCGCGAGCCA-3’ and Reverse: 5’-CACTCCACGCACGTGCCA-3’. The size of PCR product was 630 bp.
Single specific primer-polymerase chain reaction (SSP-PCR) reaction is confirmed with internal control amplification. The MOG (Myelin oligodendrocyte glycoprotein) gene was applied as internal control, which results in 356 bp fragment. Internal control primers were designed by the web primer design program, batchprimer3, which is accessible at (http://probes.pw.usda.gov/batchprimer3/). Primers were aligned in ncbi/blast. The primer sequences are: Forward: 5’-GGGACCAATTCTGTGTCACC-3’ reverse: 5’-TGAACCCAGAAGTCACTCACA-3’.
Touchdown PCR method was used to increase the specificity of primer annealing. PCR conditions are shown in Table 1.
| Step | Cycle Repeat | Function | Temperature (°C) | Time |
|---|---|---|---|---|
| 1 | 1 | Initial denaturation | 96 | 1 min |
| 2 | 5 | Denaturation | 96 | 20 s |
| Annealing | 70 | 45 s | ||
| Elongation | 72 | 25 s | ||
| 3 | 21 | Denaturation | 96 | 25 s |
| Annealing | 65 | 50 s | ||
| Elongation | 72 | 72 s | ||
| 4 | 4 | Denaturation | 96 | 30 s |
| Annealing | 55 | 1 min | ||
| Elongation | 72 | 90 s | ||
| 5 | Elongation | 20 | 1 min |
After gel electrophoresis running, random direct sequencing was used for result confirmation.
2.2. Statistical Analysis
To examine the susceptibility of HLA-A*03 allele in MS disease, χ2 was calculated and Fisher’s exact test was accomplished using SPSS for Windows V. 22.0. P value less than 0.05 was accepted as significant.
3. Results
HLA-A*03 allele was compared in 200 patients with 195 healthy controls by SSP-PCR method. Demographic and clinical profiles of MS patients and controls is illustrated in Table 2.
aValues are expressed as mean ± SD.
HLA-A*03 allele frequencies were more significant in the patient than control group (P < 0.001). Results are shown in Table 3 and agarose gel electrophoresis (PCR image) is shown in Figure 1.
| HLA-A*03 Allele | Patient, No. (%) | Control, No. (%) | P Value |
|---|---|---|---|
| + | 93 (46.5) | 51 (25.5) | < 0.001 |
| - | 107 (53.5) | 144 (73.5) | |
| Total | 200 | 195 |
We compared this allele in different ethnicities, Arab and Persian patients, and found the significant differences between them (Arab patients have more HLA-A*03 allele, (P = 0.001), since this significance not seen in healthy controls). This result is shown in Table 4.
HLA data compared with clinical categories included: disease course, EDSS, and clinical symptoms such as sensory symptoms, motor symptoms, brainstem-cerebellum, visual disturbance, urinary symptoms, and intestinal symptoms, which also showed no significant relation with HLA-A*03. Results are shown in Table 5.
| HLA-A*03 Allele | MS Patients | Controls | ||||
|---|---|---|---|---|---|---|
| Arab | Persian | Number | Arab | Persian | Number | |
| + | 40 | 28 | 68 | 26 | 17 | 43 |
| - | 20 | 47 | 67 | 72 | 61 | 133 |
| Total | 60 | 75 | 135 | 98 | 78 | 176 |
| P value | 0.001 | 0.292 | ||||
| Clinical Information | Number | A3 + | A3 - | P Value |
|---|---|---|---|---|
| Disease course | 0.235 | |||
| RRMS | 175 | 88 | 87 | |
| PPMS | 2 | 3 | 5 | |
| SPMS | 1 | 4 | 5 | |
| RPMS | 0 | 1 | 1 | |
| CIS | 10 | 4 | 6 | |
| EDSS | 0.314 | |||
| 0 | 27 | 14 | 13 | |
| 1 - 3 | 123 | 67 | 56 | |
| 3.5 - 5 | 18 | 10 | 8 | |
| +5 | 8 | 4 | 4 | |
| Symptoms | ||||
| Sensory symptoms | 80 | 43 | 37 | 0.281 |
| Motor symptoms | 86 | 39 | 47 | 0.238 |
| Brainstem-cerebellum | 48 | 26 | 22 | 0.396 |
| Visual disturbance | ||||
| Pain | 87 | 46 | 21 | 0.25 |
| Visual field defects | 46 | 26 | 20 | 0.235 |
| Urinary symptoms | ||||
| Frequency | 38 | 19 | 19 | 0.504 |
| Incontinence | 18 | 8 | 10 | 0.356 |
| Intestinal symptoms | ||||
| Constipation | 43 | 25 | 18 | 0.184 |
4. Discussion
In 1970s, linkage analysis indicated diverse variations in HLA loci, which affect the susceptibility of MS (14-16). The first discovered loci was HLA-A*03 and HLA-B7 genes (17). In the recent two decades, susceptibility loci for MS, through the HLA region, were replicated in numerous populations. Supplementary influence of class I HLA loci (HLA-A*03) doubling the risk for MS has been described (18). A study performed in Spain in 2005, indicated that frequency of HLA-A*03 allele was 41% in MS patients and 24% in health controls (18). The study performed by Harbo et al., in 2004, indicated that the combination of HLA-DR15 and HLA-A3 alleles have a high significant association with MS (19). In 2012, Kankonkar et al., reported HLA-A, B, C and DQB1, DRB1 frequency in MS patients in India and the HLA-A*03 allele indicated a significant association with MS (20).
In this study, we genotyped MS southwest Iranian patients with evaluation of HLA-A*03 significance in these patients.
We found that HLA-A*03 frequency in these patients is significantly high. Females are more susceptible for MS than males. In comparison to other studies, our study confirmed that the existence of this allele can increase the risk of MS in southwest Iranian population.
The most common symptoms of this disorder involve interruption of vision, motor and sensory systems, coordination and balance (brainstem-cerebellum), bowel/bladder/sexual, and cognition (2, 3). In this study, the effect of HLA-A*03 loci on clinical variables such as sex, course of disease, initial symptoms, and EDSS was evaluated. All of them showed no significant relation with HLA-A*03, which is in line with the study of Bayati et al., in 2008, in Iran (21).
According to the results of Sharafaddinzadeh et al., incidence and prevalence of MS is low among Arab people in comparison with the Persian (non-Arab) part of the Khuzestan province population (22), that is why we studied the HLA-A*03 in different ethnicities (Arab and non-Arab) and found a significant association between this allele and Arab patients.
We studied MS courses in these patients as a clinical symptom. This criterion is determined by the treating physician based on factors such as the number of attacks and the distances between them, during the course of the disease, the severity, and speed of progression. In this population, more than 80% of patients showed RRMS, while RPMS have less frequency, which is in line with other studies (23).
CIS is the result of only one incident of demyelination in one area, or several areas of the CNS, which lasts for at least 24 hours. Individuals who experience CIS may or may not go on to develop MS (24). From the patient population that we studied, 10 were diagnosed as CIS. We’ve followed these patients for three years and observed that all of them have been converted to RRMS.
4.1. Conclusions
Since the HLA-A*03 allele frequency is significantly high in southwest Iranian MS patients, there is probably a positive connection between this allele frequency and MS susceptibility in this population.
We observed that patients with Arab ethnicity are less than Persians, however, HLA-A*03 in Arabs are significantly more than Persians patients. Therefore, we can probably evaluate the HLA-A*03 allele as one of the MS susceptibility factors in Arabs.
In general, our purpose is to determine the simple part of the genetic profile of MS in the province of Khuzestan. We think that HLA-A*03 can be suggested as a potent genetically effective factor in the MS population.
Furthermore, more researches on additional HLA alleles and larger population are needed to recognize the role of MHC alleles in MS patients as well as find other genetic risk factors to help us identify the MS susceptibility.