1. Background
Bipolar I disorder (BD) is a complex of chronic disorder, characterized by recurring episodes of depression and mania or hypomania (1, 2). This disorder is common in different societies, with the life time prevalence of bipolar spectrum being about 1% of the general population (3-5). Bipolar disorder is the sixth disabling factor throughout the world, and in young adults it is significantly related to suicide (6). In these patients, the risk of suicide is about 15% (7, 8). Individuals with bipolar disorders have a reduced power of efficiency and productivity, thus impose high costs on the community (9-12). As suggested by the World Health Organization, bipolar disorder is the seventh cause of disability around the world (13). Bipolar disorder is predominantly diagnosed during adolescence. Overall, 25% to 55% of patients have a serious suicide attempt, which is responsible for 10% to 20% of their deaths (14). Suicide is defined as a voluntary end to one's own life. More specifically, suicidal thoughts and behaviors are classified to three categories: Suicidal ideation (refers to thoughts and plans for ending life), suicidal attempt (self-harmful behavior that leads to no fatal outcome), and completed suicide (the person’s life ends) (15-17). There is evidence of the involvement of genetic factors in suicide risk (18).
There is an increased risk of bipolar disorder developing in first-degree family and identical twins (19). Therefore, the role of inheritance in the occurrence of this disease has been proposed (20).
Although there is a strong contribution of some genetic variations to different episodes of the disease, the genetic and hereditary condition of the disease remains unclear (21), while many investigations revealed the association of some susceptibility variations with BD (2). Since most psychiatric disorders are multifactorial, finding their genetic basis is a difficult challenge. Therefore, the inheritance pattern and penetrance situation are unclear in most cases.
The genetic influence of the suicidal behavior is now widely accepted, from convincing and supportive results of family and twin studies (22). Molecular genetic studies have also been conducted with considerable extent and emphasize the role of serotonin, glutamate, dopamine, and the brain-derived neurotrophic factor related genes in the development of these disorders (23-25). Serotonin (5-HT) is one of the most important neurotransmitters involved in many personal behaviors such as attention, community behavior, appetite, and activity. It has been shown that deficiency of this neurotransmitter or its dysfunction can lead to psychological disorders (26).
Several serotonin-associated genes associated with suicidal behavior (SB) have been studied, and in this way, one of the most promising genes is Tryptophan hydroxylase (TPH), which involves the synthesis of serotonin as an important neurotransmitter and hormone (22). Tryptophan hydroxylase catalyzes the first step of serotonin biosynthesis and is a rate-limiting enzyme. Tryptophan hydroxylase enzyme is synthesized with two distinct genes, TPH1 (OMIM; 191060) located on 11p15.1 and TPH2 (OMIM 607478) located on12q21.1. Both genes are protein-coding with 70% sequence identity and different expression patterns.
So far, several studies have been conducted on the role of genes with bipolar disorders and suicide behaviors, yet conflicting results have been obtained making conclusions very difficult.
2. Objectives
The present study evaluated the association of TPH1 and TPH2 gene polymorphisms with bipolar disorder, for the first time in Sistan and Baluchistan province in Iran.
3. Methods
This was a case-control study, conducted to investigate the association of TPH1 and TPH2 gene polymorphisms with 116 patients from bipolar disorder type I, who referred to Baharan Hospital in Zahedan. Diagnosis of BD type I was performed based on diagnostic and statistical manual of mental disorders-fourth edition (DSM-IV). The patients were interviewed by a psychiatric specialist. When a patient was diagnosed as a BD type I, according to DSM-IV criteria, another psychiatric specialist had to confirm the diagnosis. Patients were entered in this study, who diagnosed as BD type I by at least two qualified psychiatrists. Patients with a history of neurological disease or another psychiatric disorder were excluded from the study. All patients were evaluated for suicidal attempt and ideation. Overall, 118 healthy age- and gender-matched healthy subjects were recruited as the control group.
Case and control groups provided informed consents, according to the Declaration of Helsinki and accepted codes of the university ethics committee.
To carry out the tests, 5 mL of whole blood was collected from each participant. Then, genomic DNA was extracted, using the salting out protocol (27). The polymorphisms of THP1 (rs1800532) and TPH2 (rs4570625) were identified using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. Table 1 contains the primer sequences used for amplification of the DNA fragments containing the polymorphisms (28).
| Polymorphisms | Sequences |
|---|---|
| TPH1(rs1800532) | f: 5’-CGTCCTGTGGCTGGTTACTT-3’ |
| r: 5’-CACGCTGCAGTGCTTAACAT-3’ | |
| TPH2 (rs4570625) | f: 5’-TTTTATGAAAGCCATTACACAT -3’ |
| r: 5’-TTCCACTCTTCCAGTTATTTTA-3’ |
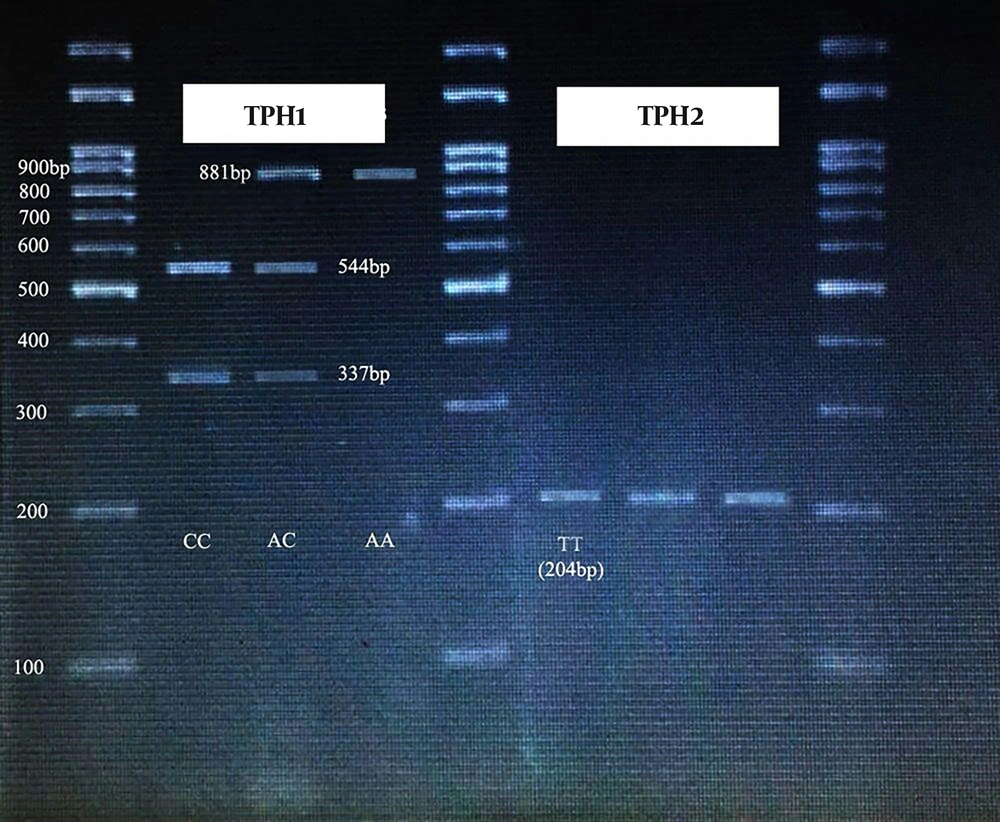
The annealing temperature for TPH1 (rs1800532) was 56°C and the PCR products were digested by NheI restriction enzyme for two hours at 37°C. The PCR product size was 881 bp, after digestion, the allele A was uncut while the allele C was cleaved to fragments of 337- and 544-bp.
The annealing temperature for TPH2 (rs4570625) was 51.9°C, and NheI restriction enzyme (four hours at 37°C) was used for determining the polymorphism alleles. The PCR product size was 204 bp. The digested products contained two fragments of 55 and 149 bp for allele G, while the allele T was uncut.
Data obtained from the distribution of genotype and phenotype of patient and control groups were analyzed using the statistical software SPSS 18 (SPSS, Chicago, IL) and applying independent sample 𝑡-test, chi-square (χ2) test and odds ratio (OR) with 95% confidence intervals (CI). P values of less than 0.05 were considered statistically significant. Deviation from Hardy-Weinberg equilibrium in cases and controls of this study was also evaluated. To confirm the results, three samples of each genotype were sequenced in forward and reverse directions, using the same primers of PCRs by an ABI Prism 3700 instrument (Applied Biosystems, Foster City, CA).
4. Results
In this case-control study, 116 BD patients (42% female) and 118 healthy controls (41% female) were recruited. The mean age of the subject group was 29.5 (± 9.8) years and the control group was 33.4 (± 9.7) years. Case and control groups were adjusted for age and gender. In the case group, 71% were married, 17.3% were illiterate while 3.6% of them had academic education. Among them, 24.5% had suicidal ideation and 12.7% had suicidal attempt.
Genotype frequencies for polymorphisms of TPH1 (rs1800532) and TPH2 (rs4570625) held the Hardy-Weinberg equilibrium (P > 0.05).
Based on the calculated P value and odds ratio for the genotype frequencies of rs1800532 of TPH1 gene, only CC genotype showed significant differences among the BD patients and healthy control group (P = 0.04, OR = 0.5, 95 % CI in 0.2 - 1). None of the allele frequencies showed a significant difference between the two groups.
The allelic and genotype frequencies for TPH2 gene polymorphism (rs4570625) did not show any significant difference between patient and control groups (P > 0.05). The data are shown in Table 2.
| Variables | Casea (N = 116) | Controla(N = 118) | P Value | OR (95% CI) | Power Study |
|---|---|---|---|---|---|
| TPH1 | |||||
| AA | 28 (24) | 25 (21) | 1 | ||
| AC | 70 (60) | 57 (48) | 0.8 | 1.1 (0.6 - 2.1) | 54.2 |
| CC | 18 (16) | 36 (31) | 0.04 | 0.5 (0.2 - 1) | 87.7 |
| Allele | |||||
| A | 126 (54) | 107 (45.3) | 1 | ||
| C | 106 (46) | 129 (54.7) | 0.06 | 0.7 (0.5 - 1) | |
| TPH2 | |||||
| TT | 116 (100) | 118 (100) | 1 | ||
| TG | - | - | - | - | |
| GG | - | - | - | - | |
| Allele | |||||
| T | 232 (100) | 236 (100) | 1 | ||
| G | 0 (0) | 0 (0) | - | - |
aValues are expressed as No. (%).
The sequencing results of the three samples of each genotype confirmed the RFLP results.
Figure 1 shows the gel electrophoresis of TPH1 and TPH2 gene polymorphism.
5. Discussion
As psychiatric disorders occur in different backgrounds, genetic differences in individuals can be considered as an influential issue (29). Gene polymorphism, while being a normal variation, can have an effect on gene function by influencing gene expression. Since serotonin plays a very important role in social behavior and mood, the serotonergic system is a candidate in psychiatric research. Tryptophan hydroxylase (TPH) is the main enzyme in the synthesis of the neurotransmitter serotonin, which is coded by two separate genes, TPH1 and TPH2. These two isoforms have 70% sequence identity with different expression patterns, yet both are responsible for biosynthesis and rate-limiting of serotonin. TPH1 is the predominant isoform in the periphery, while TPH2 is in the central nervous system (CNS) (30).
TPH1 gene polymorphism is a subject of many kinds of research. One of the most known variations in this gene is A218C (rs1800532) located in intron 7 (31). In most cases, the variation in intron does not affect the structure in protein coding. However, A218C is situated on a transcription factor binding site (GATA-1), which strongly affects the expression of some genes (32), thus it could be considered as a functional SNP. Some studies have shown that allele A of A218C has an important role in TPH1 gene expression (33). This allele showed a positive association with suicide (34). It is still unclear how much the expression of TPH1 gene affects CNS estrogen function, yet it affects serotonin levels (33). However, it has been mentioned that the variations of intron 7 and 8 in TPH1 gene have a great association with major depression (35). As shown in Table 2, positive association with BD was found in CC genotype (P values = 0.04; OR = 0.5, 95% CI in 0.2 - 1) for TPH1 gene polymorphism (rs1800532), yet not for other genotypes and alleles. In a study, the CC genotype was associated with suicide attempt only in individuals more than 65 years old (36). However, it did not happen in this study and this association was not age-related.
TPH1 A218C polymorphism is known to be associated with many disorders, such as borderline personality disorder (BPD) in the USA (31), anger-related personality and suicidal behaviors (22, 34, 37-39), suicide in Turkey (40), schizophrenia (41), bipolar disorder in Taiwan (42, 43), Poland (44) and Caucasian population (45) and depression in Finland (46). However, many studies found no association between TPH1 A218C polymorphism with mental and behavior disorders in Germany and China (47, 48), suicide in Sweden (49) and Denmark (50). Therefore, there are many contradictions in this area.
TPH2 gene (12q 21.1), which was discovered after TPH1 gene, is called brain-specific isoform and carries out its main activity in neural tissues (51). TPH2 gene is expressed much more than TPH1 in the brain, thus it is suggested that it has a more important role than TPH1 in the brain. Different studies have shown that variations in this gene are associated with bipolar disorder, major depression and suicidal attempt (52-57), ADHD (58), autism (59), and schizophrenia (60).
The current study showed no association between the TPH2 gene polymorphism G-703T (rs4570625) and bipolar disorder, neither genotypes nor alleles.
Obviously, genetic variations that affect the function of TPH2 gene or its expression, could be associated with the mentioned diseases. The polymorphisms located in promoter and 5'-regulatory region of THP2 gene are the subject of many types of research (61, 62). G-703T (rs4570625) is a variation in the promoter of TPH2 gene and is reported to be in association with many disorders such as ADHD (63, 64).
It is suggested that G-703T down-regulates the in vitro expression of TPH2 gene, so it may have a role in serotonin function in the brain (61, 65).
According to studies on the role of TPH1 gene in different diseases, there are contradictions with the TPH2 gene’s relationship with diseases (66).
Many studies revealed the lack of association of TPH2 gene variations with suicide (22, 67), BD, and schizophrenia (60, 68, 69). However, a number of studies admit the relationship between polymorphisms of TPH2, especially rs4570625, with major depression (70), suicide (71), and bipolar disorder (51).
Although the role of TPH2 gene in bipolar disorder is ambiguous, it is considered as a related gene to bipolar disorder (72, 73).
To the best knowledge of the authors, this was the first study on the relationship of TPH genes with bipolar disorder in Iran. However, there is a long way to find the exact genetic basis of bipolar disorder, to find the real basis of the disease.