1. Background
Parkinson’s disease (PD) is an incapacitating chronic brain disturbance, which is accompanied by progressive destruction of dopaminergic neurons in Substantia Nigra pars compacta (SNc) (1-3), leading to dopamine (DA) depletion in nigrostriatal tract and loss of dopaminergic fibers in this neuronal pathway (4).
Despite extensive studies on PD, the precise etiology and mechanisms involving in loss of dopaminergic neurons are unknown (1). However, it has been declared that PD may result from multiple pathogenic factors such as neuroinflammation, reactive oxygen species (ROS) production, mitochondrial damage, nitric oxide generation, and misfolded protein accumulation (5). A pieces of evidence suggested that neuroinflammation and oxidative stress (OS) were the two key events involved in the death of dopaminergic neurons and played a critical role in the PD (6). Owing to dopaminergic neurons contain iron and the amount of ROS is increased due to dopamine metabolism, antioxidant system is necessary (7). In addition, neuroinflammation and mitochondrial dysfunction involve in the generation and increment of ROS and other free radicals and cause oxidative damage; consequently, neuronal cell death in SNc and loss of nigrostriatal fibers occur through this process (7).
Exposure to environmental factors is one of the key factors, which have a close relationship and plays a role in PD occurrence (8). Rotenone (ROT) is a pesticide and herbicide derived from leguminosa plants and nowadays, is widely used in cultivating. The ROT, due to highly lipophilic properties, conveniently passes the blood-brain-barrier and enters to DA-ergic neurons without needs to any transporter unlike other toxins, including parquet, MPTP, and 6-hydroxydopamine (9-11). Classically, the ROT damages the oxidative phosphorylation in mitochondria by hindering the complex Ι of the mitochondrial electron transport chain and causes the production of ROS, neuroinflammation, and microglia activation (10). Then ROS production and OS induced by ROT, selectively generate the death in DA-ergic neurons (12). This neuronal degeneration couple with neuroinflammation result in microglia activation (12, 13). Recent animal model studies confirm that ROT can induce neurotoxicity and mimic PD classical symptoms such as movement impairments, tremor, and rigidity in patients with PD (14, 15).
Human and animal experimental studies of PD suggests a significant role of the neuroinflammatory process in the occurrence of this neurological disorder (16). Cyclooxygenase-2 (COX-2) is an essential enzyme in inflammatory procedures in the body that is synthesized in response to pro-inflammatory cytokines and agents. It is the first enzyme, which converts arachidonic acid to prostaglandins (markedly PGE2) and thromboxanes (17). The COX-2 is constitutively expressed in SNc neurons of the central nervous system, where it is responsible for neuronal plasticity and integrity of signal transmission (17). Moreover, another study showed that COX-2 overexpression played a detrimental role in neurodegeneration disease (16). Experimental evidence showed that COX-2 inhibitors could have a neuroprotective effect in different neurological impairments such as ischemia and epilepsy (18, 19). Celecoxib (CLX) is a selective COX-2 inhibitor, and its neuroprotective effects have been established in several experimental studies (20). Kaizaki et al. reported that CLX could decrease the dysfunction of brain DA-ergic neurons in neonatal rats (20). Fan et al. in their study showed that CLX attenuated inflammation of brain damages caused by exposure to systemic lipopolysaccharide (21). Another study -related to our research team regarding CLX effect on CA1 hippocampal neurons and OS in ROT-induced rat model of PD- revealed that treatment with CLX, due to a decline in apoptosis of CA1 hippocampal neurons, caused an increment in the passive avoidance memory, and markedly reduced MDA levels, while increased TAC (22).
2. Objectives
The aim of this study was to evaluate the effects of celecoxib (CLX) on ROT-induced rat model of PD. To this aim, the suppression of neuroinflammation and oxidative stress-mediated apoptosis was surveyed.
3. Methods
3.1. Animals
A total of 32 Sprague-Dawley male rats with an average 300 - 360 g body weight and 20 weeks aged were purchased from the animal house of Medical College, Zahedan University of Medical Sciences (ZAUMS). The animal was treated in the standard cage (ventilated polypropylene) with 25°C, 12/12 hours light, dark cycles, and enough food and water during four weeks of the study period. All the animal experiments were conducted according to international guidelines for animal experiments that were approved by the Research Ethics Committee of ZAUMS.
3.2. Experimental Design
In this experimental study, the animals were randomly classified into 4 equal groups with 8 rats in each one as follows: control group, sham group received 1 mL distilled water orally + corn oil subcutaneously (s.c); PD group only received 2.5 mg/kg/48 hours ROT s.c; CLX group received 20 mg/kg/24 hours CLX treatment via gavage + ROT. Celecoxib was purchased from Razak Laboratory Co., Tehran, Iran. At the end of the experiment, the animals were sacrificed and their brains were removed and stored in the buffer. Then the samples were transferred to the laboratory for biochemical and histological assessments. All of the experimental design in the current study was approved by the Care of Animal House Ethics Committee of the ZAUMS, Zahedan, Iran (code number: IR.ZAUMS.REC.1395.30).
3.3. Tissue Sampling and Nissl Staining
To tissue sampling, half of the rats following the anesthesia were perfused transcardially by 4% paraformaldehyde fixative. The brains of animals were quickly removed and were cut and immersed in post-fixative (10% formaldehyde) for two weeks. Then the routine tissue processing was done; 7 μm thickness at serial coronal sections of the brain were cut (bregma-4.52 mm to -6.04 mm) with a 30 μm interval between each consecutive section for Nissl staining (15). The coronal sections were stained with 0.1% cresyl violet and finally mounted. Viable dopaminergic neurons (the cells with an obvious nucleus) counted in five coronal sections of SNc from each animal in five separate microscopic fields randomly using a light microscope at 400X magnification (23).
3.4. Biochemical Analyses
3.4.1. Ferric Reducing Antioxidant Power (FRAP) Assay
The total antioxidant capacity (TAC) of the midbrain was determined with the Benzie and strain method (24). A potential antioxidant will reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+), and the latter forms a blue complex (Fe2+/ TPTZ), which increases the absorption at 593 nm. In brief, a FRAP’S working solution was prepared by mixing 10 volumes of buffer acetate (0.3 M, pH = 3.6) with 1 volume of trypyridyl-s-triazine (TPTZ, 10 mM) solution in HCl (40 mM); then 1 mL FeCl3 solution (20 mM/L) was added and mixed. Subsequently, 1.5 mL of reagent was added to 50 μL of 10% midbrain homogenate and heated for 10 minutes at 37°C. The complex between TPTZ and Fe2+ resulted in blue color with absorbance at 593 nm (25). Data were presented as μmol/g of wet tissue.
3.4.2. Malondialdehyde (MDA) Activity Assay
Malondialdehyde (MDA), as an index of lipid peroxidation, was measured by Ohkawa’s method (26). In brief, 0.2 mL midbrain homogenate, 1.5 mL acetic acid (20%), 0.2 mL sodium dodecyl sulphate (8.1%), and thiobarbituric acid (1.5 mL, 0.8%) were mixed in a 10 mL centrifuge tube. Then the admixture was warmed in a bain-marie (95°C, 60 minutes). After cooling, 5 mL of n-butanol: pyridine (15:1 %v/v) was added. Later of centrifugation, the supernatant absorption was estimated at 532 nm (25). The MDA level was presented as nmol/mg of protein.
3.5. Statistical Analyses
All of the data were expressed as the mean ± SEM. To measure statistical differences between the experimental groups ANOVA and Tukey post-hoc tests were applied using SPSS software version 16. The statistical significance level was considered P value less than 0.05.
4. Results
4.1. Effect of CLX on TAC Levels
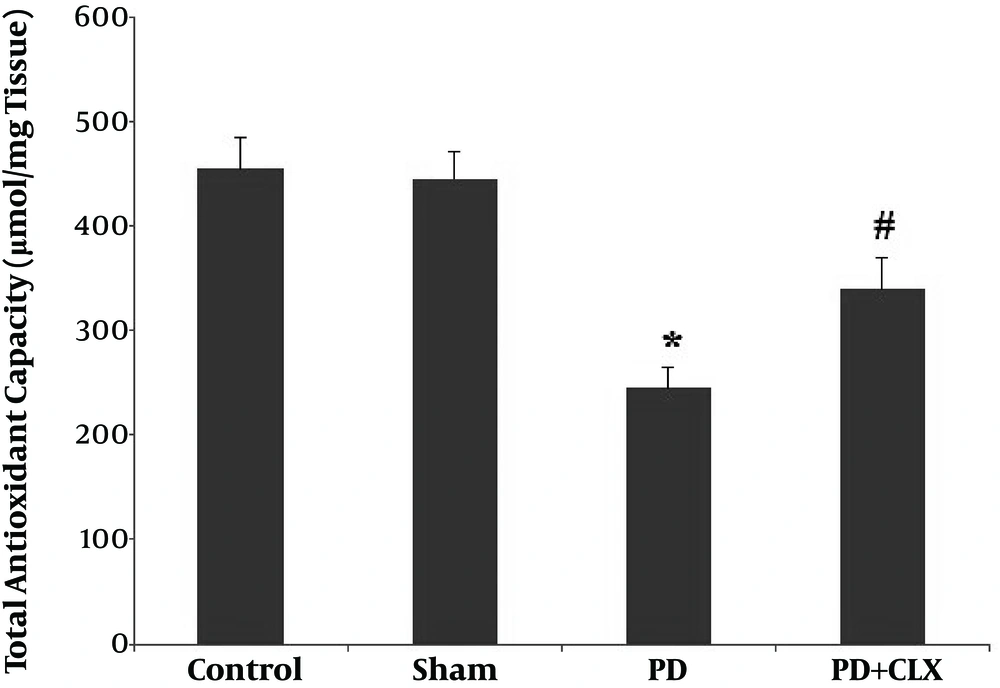
A significant reduction (P < 0.001) in TAC level was found in the ROT group compared with the control and sham groups. Treatment with CLX markedly increased the TAC level in comparison to the ROT group (Figure 1).
4.2. Effect of CLX on MDA Levels
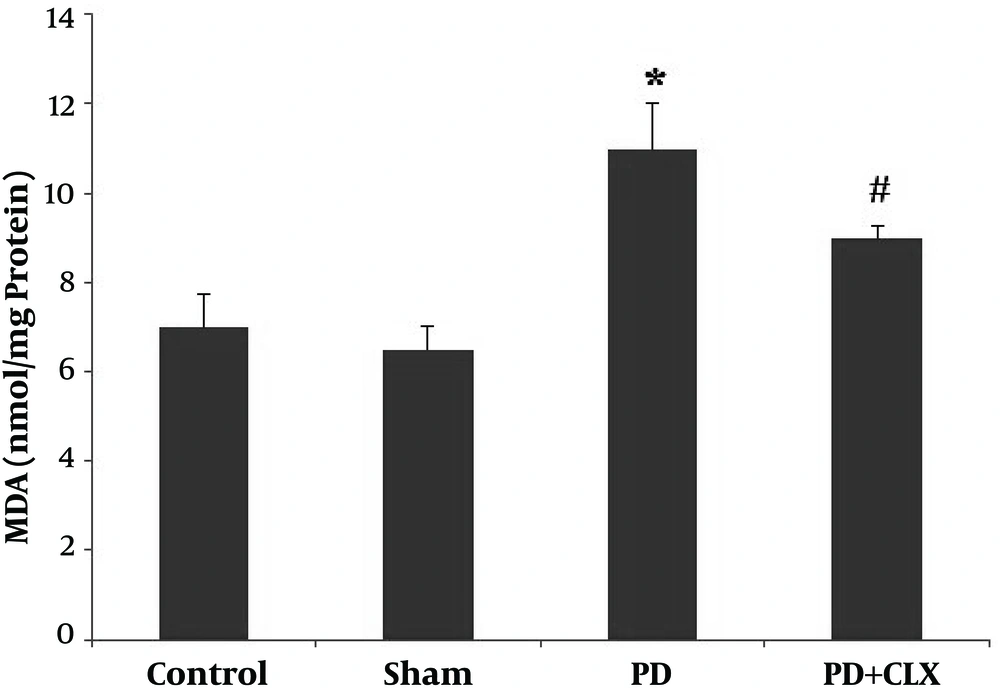
Figure 2 shows PD induction elevates the MDA level in SNc (P < 0.001) compared with the control group. Treatment with CLX significantly diminished the MDA level compared with the ROT group.
4.3. Histological Assessment
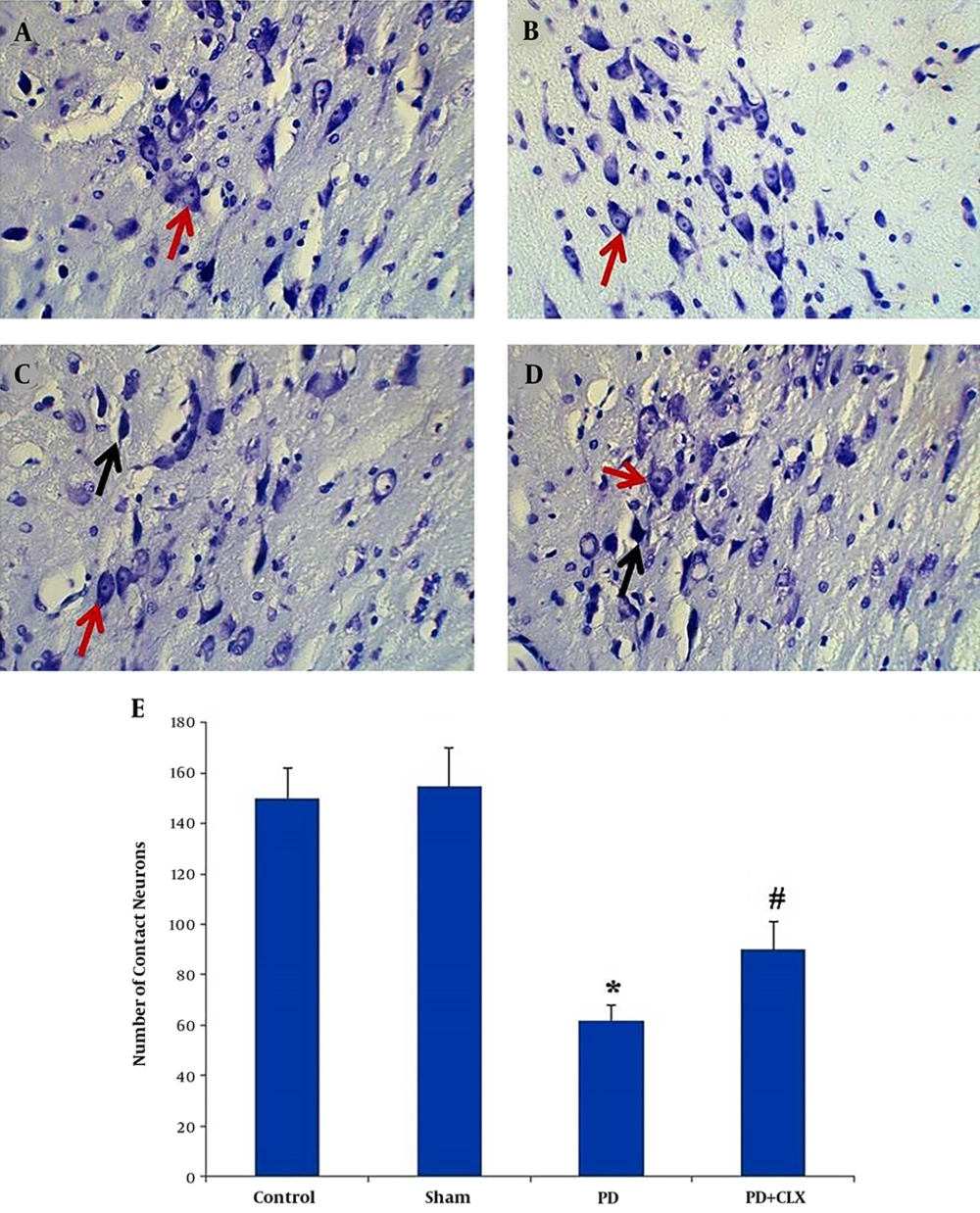
Nissl-stained intact DA-ergic cells in the SNc area were counted in all studied groups. According to observations, the nuclei of viable neurons were intact in the control and sham groups (Figures 3A and 3B). Interestingly, most DA-ergic cells in SNc of ROT group had pyknotic and dark appearance, which were entirely degenerated. The number of intact Nissl-stained DA-producing cells was significantly lower in comparison to those of the control and sham groups (P < 0.001) (Figure 3C). Moreover, according to the Nissl staining, the number of viable DA-producing neurons in the SNc of the CLX group was significantly increased in comparison to the ROT group (Figures 3D and 3E).
Effects of celecoxib on dopaminergic cell count in the midbrain SNc of the rats with PD (Nissl staining). Viable dopaminergic neurons were quantified at 400X magnification. Red arrows show the viable cells and black arrows show the pycnotic cells. The graph shows the number of intact dopaminergic neurons. A significant decrease (*P < 0.001) was observed in the number of contact neurons in the PD group compared with the control group, whereas a significant increase (#P < 0.01) was observed in the number of contact neurons in PD + CLX group compared with PD group A: control group, B: sham group, C: PD group, and D: PD + CLX group.
5. Discussion
The hypotheses of our study were that the oxidative damage and neuroinflammation arising from subcutaneous injection of ROT are able to induce neuronal death and imbalance of antioxidant activity in rat brain cortex through a neurodegenerative process. Also, we guessed that CLX treatment as a COX-2 selective nonsteroidal anti-inflammatory drug would effectively reduce OS and thereby significantly attenuates ROT-induced brain cortex apoptosis.
The findings of the current study demonstrated that CLX treatment had neuroprotective effects on DA-ergic neurons of SNc by improving the TAC and reducing the lipid peroxidation; consequently, it inhibits OS and neuroinflammation-mediated apoptosis. The PD is characterized by selective degeneration of DA-ergic neurons in the SNc that occurs due to many critical processes, including OS, neuroinflammation, and programmed cell death (Apoptosis) (27). The OS has currently been proposed as a substantial process involved in the pathogenesis of neurodegenerative disorders. The evidence obtained from clinical and experimental studies demonstrated that in neurodegenerative diseases such as PD, there are high levels of OS biomarkers and lower levels of endogenous antioxidant enzymes in the brain tissues, which selectively affects DA-producing neurons (28).
Our previous study results regarding ROT-induced model of PD in rat showed that ROT disrupted antioxidative defense balance system via inducing OS and increased lipid peroxidation in DA-ergic neurons. Furthermore, the results demonstrated that improvement in antioxidant biomarkers protected from depletion of the tyrosine hydroxylase-positive neurons in the midbrain (15). Teismann et al. stated that COX-2 levels were increased in DA-ergic neurons of the brain in MPTP mice due to selective neuronal death. Their study results provided evidence that COX-2 inhibitors do not protect versus MPTP-induced DA-ergic degeneration by relieving inflammatory process rather than COX-2 inhibition hampers the ROS dopamine-quinone formation (16). Despite extensive studies in the last decade, the role of COX-2 in inflammatory and neurodegenerative processes or its beneficial or harmful effects are still contradictory (29). Reksidler et al. showed that treatment with COX-2 inhibitor provided a neuroprotective effect in MPTP-lesioned rats and this strategy improved motor and cognitive functions in the early phase of PD (30). The results of the present study regarding CLX effects on biochemical and the numbers of Nissl-stained neurons in the SNc indicated an improvement in TAC, reduction in lipid peroxidation (MDA level), and a significant increase in the number of intact neurons in the brain tissue of the CLX group in comparison to the PD group. These findings support this view that COX-2 has a crucial role in the PD and reduces DA-producing neuron death in the SNc. Thus it seems that consumption of selective COX-2 inhibitors such as CLX can exert its neuroprotective effects in ROT model of PD.
Neuroinflammation has been implicated as one of the factors that play potential roles in the PD underlying mechanisms. Microglial cell activation by inflammatory agents may involve in the neurodegenerative process via the release of pro-inflammatory cytokines, synthesis of ROS and nitric oxide, and structural invasion, which have harmful impacts on DA-ergic neurons (13). Moreover, Choi et al. claimed that neuroinflammation subsided by anti-inflammatory drugs attenuates degeneration of DA-producing neurons (31).
Anusha et al. findings revealed that MDA activity was significantly increased in the SNc of ROT-induced PD in rat compared with the control group. Also, the activity of antioxidant biomarkers in the striatum was significantly declined in the ROT-induced PD group compared with those of the controls. In addition, their findings demonstrated that apigenin with anti-inflammatory properties effectively scavenging free radicals, and relieved the OS applies its neuroprotective effects via control neuronal apoptosis by neurotrophic activity in ROT model of PD (27).
In conclusion, according to these results, CLX treatment can effectively improve the antioxidant defense system and attenuates striatum disturbance in ROT-induced rat model of PD. These findings suggested that CLX plays a neuroprotective role by inhibition of OS and neuroinflammation. The CLX protected ROT-induced DA-ergic neuron apoptosis and histological changes through positively regulating the enzymatic antioxidant system and inflammatory process. Further studies are needed to clarify the effect of CLX on DA-ergic neuron function and DA levels in the SNc.