1. Background
Vaginitis is one of the most common diseases of women, which is important in health centers around the world and requires health care (1).
About 10 million health centers annually address vaginal discharge complaints. Vaginal symptoms are usually related to bacterial vaginitis, vulvovaginal candidiasis, and trichomoniasis, in addition, Cholamydia trachomatis and Nisria gonorrhoea are other causes of vaginitis (2).
Trichomonas vaginalis is a flagellate protozoan transmitted through sexual contact (3). It is also the only pathogenic form of the parasite in humans, this parasite grows in a humid environment, pH ranges from 4.9 to 7.5 and temperatures ranging from 35°C to 37°C. If these conditions turn less or more than optimal, the organism disappears. Women usually have vaginal and urethral infection, however, the parasite may also infect the cervix, the Bartholin glands, or the bladder. In men, the organism is often found in the extremities of the urethra, however, it involves the prostate gland, seminal sac, and epididymis rarely.
The parasite causes various diseases like vaginitis, urethritis, and prostatitis (4).
This parasite has a 15% - 10% prevalence and is one of the most common sexually transmitted diseases after bacterial infection of Chlamydia. With the report of the World Health Organization, 170 - 190 million people worldwide are now infected with this parasite, with 120 million cases of trichomoniasis annually, by checking the results; we find that nearly 20% of cases show vaginitis symptoms.
According to a recent systematic study, the prevalence of trichomoniasis in Iran is estimated at 8% (5).
Prevalence increases by up to 30% in high risk populations (6). It should be noted that pregnant women with trichomoniasis may show multiple side effects, including preterm labor, low birth weight, premature rupture of the membrane, tubal infertility, and atypical pelvic inflammatory disease. On the other hand, increasing the transmission risk of HIV/AIDS, human papillomavirus, and cervical cancer are another serious complication of this disease (7). Men with Trichomonas vaginalis are usually unmarked and show symptomatic infection that is associated with urethritis (urticaria and secretion) (4). Trichomoniasis in men is usually treated without medication (8). This wide spread use of herbal medicine can be due to various reasons, such as fewer side effects, better patient acceptance due to traditional medicine advice, lower prices for medicinal plants, and compatibility with the physiological function of the human body. Herbal medicine is a branch of traditional medicine in ancient civilizations like Iran, which played a major role in the treatment of diseases one century ago.
Candidiasis is one of the most common opportunistic fungal diseases in humans. This disease caused by the yeast fungus is called candida (9). Candida albicans yeasts are commonly found in humans, and their growth is typically limited by the human immune system and other microorganisms, such as bacteria that occupy identical places in the human body. Candida infection shows a range of surface complications such as oral thrush and vaginal inflammation that potentially treats by itself in humans (10).
Candida’s genus contains a heterogeneous group of organisms that grows in yeast, and most members of this genus produce false filaments during their growth; however, Candida albicans and Candida Doblini Ninci have the true form of Heif and cells with thick walls; both of them produce Chlamydosporum, which are detectable in a laboratory.
Candida species are the fourth cause of blood infections in hospitalized patients and they can potentially cause the mortality of patients that have been admitted to hospitals in the United States (11).
However, there are also limitations in the treatment of fungal diseases, such as the prevalence of resistant species to the antifungal agents and their side effects. Therefore, studying on new drugs that have fewer side effects for patients at the same time with eliminating or inhibiting the pathogen is necessary. For this suggestion, studying on medicinal plants is important.
Rosemary (Rosmarinus officinalis L.) is a plant from the family of mint, which is native to the Mediterranean area, and also in Iran.
2. Objectives
We are familiar with anticancer, anti-inflammatory, antimicrobial, and antioxidant effects of this herb (12). The main purpose of this study is to investigate the effects of methanolic extract of Rosmarinus officinalis on Trichomonas vaginalis and Candida albicans under laboratory conditions.
3. Methods
3.1. Extract Preparation
The massage method was used to prepare extracts. In this way, after crushing the leaves of the plant, 50 g of each sample was embedded in methanol for 48 hours, and after this time a filter is used for material separation then, the extracts were concentrated by using a rotary machine (vacuum distillation) at 40°C to 50°C, and finally, dried at 40°C for two days.
The Rosmarinus officinalis plant used in this research was collected from the city of Zabol and was diagnosed by Rosemary officinalis by the expert of Zabol National University, according to the visual characteristics and herbalists’ descriptions. After collecting the leaves, the leaves were dried in the shade in order to prepare the extract with crushed mill. Then, vaginal discharge of women with vaginitis symptoms was observed directly in the treatment centers. After confirmation, Trichomonas parasite was cultured in a media and prepared eggs. Five tubes containing culture media and T. vaginalis were considered as controls. Five tubes containing metronidazole and T. vaginalis, DMSO and T. vaginalis, and dry extract at concentrations of 0.1, 0.01, 0.001, 0.0004, 0.0002, and 0.0001 mg/mL in DMSO solvent and T. vaginalis were prepared in a 37-degree incubator.
3.2. Isolation of Candida albicans
Samples were taken by two sterile swabs of vaginal discharge. Samples were taken in pipettes containing 2 mL of sterilized distilled water while transferring to a laboratory. The swabs were used for direct testing with 15% potassium, cultured on Sabouraud dextrose agar (Merck, Germany) and chromogen agar (France, France). The culture media was incubated for 48 to 72 hours at 30 degrees Celsius to confirm the diagnosis of the albicans species from other species.
Candida from the colonies on the corn-mili agar + tween media linear cultures was given. In addition, tuberculosis and beta-glucosidase tests were used to differentiate Candida albicans from other species. The plates were placed at 25°C for 72 hours. From a fresh culture, single colonies of each yeast were transferred to a solution of 20% water-glycerol and the sample was transferred. They were kept at a minimum temperature of 20°C, the samples were stored in the solution of 20 g/g glycerol incase it was needed to repeat each step of the experiment.
3.3. Disc Diffusion Method Was Used for Determining the Diameter of the Extract Halo on Candida albicans
Agar diffusion method and disc diffusion method were also used to determine the antifungal activity of medicinal plants extract against opportunistic fungies (13). It should be noted that the agar diffusion method was subjected to minor changes (14).
In this method, according to the relevant standard, after accurate counting of fungi and preparation of standard suspensions with a certain amount of fungi, it was transferred into a media and cultured using sterilized swabs. Then, the wells with a diameter of 5 mm were placed on a subrock dextrose agar media, and the ends of each well were closed to a substrate in sterile conditions, which resulted in preventing the extract from being extracted below the culture media. Next, extract in these wells that have different concentrations of 2.5 - 40 µg/mL were sold and incubated at 37°C, after 24°C - 48°C. The non-growth halo diameter was investigated.
3.4. Determination of the Minimum Inhibitory Concentration of MIC Extract on Candida albicans
To determine the MIC for Rosmarinus officinalis extract separately, a series of 9 tubes are used to test the dilutions of each extract and a tube as a positive control containing diluted extract and culture media and negative control including microbial suspension and culture media. We considered that the initial concentration of the extract is 50 mg/mL, which is obtained by inserting 1 mL of the extract into the first tube containing one ml of culture media at a concentration of 25 μg/mL. In this way, for the first tube, one milliliter of the extract is diluted at a concentration of 100 μg/mL with one mL of the dextrose broth agar media, and, similarly, taking 1 mL of the first tube, and pouring it into the second tube containing one milliliter The litter is Dextrose Broth Agar, which is transmitted to the last tube. The tube was lifted out of one milliliter and poured out. By doing this process, the dilution of each tube was half the dilution of the tube before. A total of 50 μL of microbial suspension was transferred into all the tubes except the positive control tube. The dilutions of the extracts for our studied fungi were performed in a completely separate manner. The series of test tubes were placed in an incubator for 24 hours at 37°C, then, the tubes were examined for turbidity due to growth of the fungus.
The results were expressed as mean or ranked in order of importance as percent. The data were subjected to one-way analysis of variance (ANOVA), using the SPSS V. 17 software. The P value of less than 0.05 were regarded as significant.
4. Results
The results of this study showed that in the concentration of 0.0001 at 4 hours, the parasite Spherical and inactive, dead. At a concentration of 0.0002 in 2 hours, the parasite is spherical and inactivated (Table 1).
| Extract Concentration | At the Time of Cultivation | Hour 1 | Hour 2 | Hour 4 |
|---|---|---|---|---|
| 0.1 | Dead and without walls | - | - | - |
| 0.01 | Dead and without walls | - | - | - |
| 0.001 | Spherical and inactive | - | - | - |
| 0.0004 | Live with low mobility and dead | - | - | - |
| 0.0002 | + | Live with low mobility | Spherical and inactive | - |
| 0.0001 | + | + | Live with low mobility | Spherical and inactive, dead |
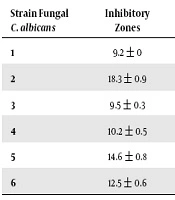
The results of this study showed that Rosmarinus officinalis extract in concentration of 100 µg/mL has an inhibitory effect on Candida albicans, therefore, the highest inhibitory diameter was 18.3 ± 0.9 mm while two other strains exhibit resistance and no inhibitory zones (Table 2).
| Strain Fungal C. albicans | Inhibitory Zones | Strain Fungal | Inhibitory Zones |
|---|---|---|---|
| 1 | 9.2 ± 0 | 7 | 17.4 ± 0.5 |
| 2 | 18.3 ± 0.9 | 8 | - |
| 3 | 9.5 ± 0.3 | 9 | 9.6 ± 0.5 |
| 4 | 10.2 ± 0.5 | 10 | - |
| 5 | 14.6 ± 0.8 | 11 | 15.3 ± 0.5 |
| 6 | 12.5 ± 0.6 | 12 | 12 ± 0.8 |
5. Discussion
Trichomonas vaginalis acts as a predisposing factor in the transmission of human immunodeficiency virus and other sexually transmitted infections (15). According to numerous reports about drug resistance of Trichomonas vaginalis to metronidazole, which is the main treatment of this infection, it is needed to find an effective drug with minimum complications (16).
Candida albicans is an eukaryotic fungal pathogen that causes diseases like oral thrush and vulvovaginitis. Many of its biological pathways are common with humans, and most antifungal drugs have different side effects at different doses. Despite the effect of these drugs on the patient’s recovery, excessive use of drugs caused drug resistance, which has led researchers to use plant compounds as an alternative for antibiotics to inhibit the growth of these pathogens in research’s.
Rosmarinus officinalis is used as a styptic agent, antipyretic, antispasmodic, and sudorific in traditional medicine. Extract and volatile oil are used for abortion and to increase menstrual bleeding. The use of rosemary extract in cosmetics is very common, and evidence suggests the effect of its lotion on stimulating hair growth and prevention of scabies. Historical reports of rosemary treatment are available as herbal medicine. Rosemary is one of the oldest medicinal plants known to have been used centuries ago to strengthen memory and brain activity.
Major components of the volatile oil include: Camphor, Borneol, α-Pinen, the amount and percentage of each of these materials depends on the environmental conditions of plant’s site.
Other ingredients in Rosemary leaf include: luteonolin, jenkanin, tannin, resin, pasonin, fat, carbohydrates, and vitamins.
The results of this study showed that Rosmarinus officinalis has good anti-fungal and anti-parasitic activities.
In another study, the results showed that Rosmarinus officinalis essential oil inhibits bacteria including E. coli, Bacillus cereus, Staphylococcus aureus (17), Clostridium perfringens, Aeromonas hydrophila, Bacillus cereus, and Salmonella choleraesuis.
Essential oils of Zataria multiflora and Myrtus communis showed inhibitory effects at concentrations of 0.1%, 0.01%, 0.001%, and 0.004% on Trichomonas vaginalis (18). Meanwhile the methanolic extract of Myrtus communis showed an inhibitory effect at concentration of 0.1 mg/mL (19).
In another study, aqueous extracts was also tested, Verbena sp. (Guachu ka’a in Mbya-Guarani language) and Campomanesia xanthocarpa (Guavira in Mbya-Guarani language) showed that the highest activity against T. vaginalis with MIC value of 4.0 mg/mL reached 100% of efficacy against the parasite. The kinetic growth assays showed that the extracts promoted complete growth inhibition after 4 hours of incubation (20).
Plant of Verbena sp, C. xanthocarpa, Myrtus communis were tested against T. vaginalis, however, without success (21, 22).
In the study of Hassani et al., the effect of hydroalcoholic extract of eucalyptus in comparison with metronidazole on Trichomonas vaginalis has been evaluated under laboratory conditions. Extract of E. camaldulensis showed 80% growth inhibition (GI) in a concentration of 12.5 mg/mL during 24 hours. Diethyl ether extract in a concentration of 25 mg/mL showed 100% GI during 24 hours. With ethyl acetate extract, 100% GI was detected with the minimum concentration of 12.5 mg/mL in the first 24 hours. Finally, water extract in a concentration of 50 mg/mL showed 80% and 100% GI after 48 and 72 hours, respectively (23).
Amaryllidaceae species showed a promising activity against Trichomonas vaginalis (24, 25).
Also, different parts of the plants (leaves, flowers, etc.) also have antimicrobial properties (26).
In a study by Nejati et al., on 75 Wistar male rats (weighing 210 ± 10 g), after general anesthesia, a 1.5 to 1.5 cm squared sore wound made and immediately implanted and infected by Candida albicans. Experimental rats were randomly assigned into three groups of 25 (control and groups treated with 1.5 and 3% oat) and each group was divided into 5 subgroups of 5 (sampling groups in different days). During the course of the project, at the end of days 4, 8, 12, 16, and 20, wounds were collected from different groups for pathological examination by a special punch for biopsy. The results showed that application of 1.5% and 3% ointment from rosemary essential oil significantly reduced the infection rate and increased collagen content and production of coated tissue compared with the control group. Based on the results, the wound healing process in the ointment was 3% lower than the lower dose and the control showed a better result (27).
In the Natanzian Ghahfarkhi et al. study, the antifungal effects of essential oils and alcoholic beverages on isolates that are clinically resistant and susceptible to fluconazole Candida albicans were examined in vitro, the inhibitory effect of alcoholic and oleaginous extracts of herbs on the growth of susceptible and resistant strains to fluconazole Candida albicans was confirmed (28).
In the study of Jafari et al., the concentration of 8.75 mg/mL from the extract of Angus spp. completely prevented the growth of Candida albicans and destroyed all the live Candida cells at this concentration (MFC). In addition, concentrations of 4.23 mg/mL and 4.4 mg/mL aqueous extract were obtained as minimum inhibitory values of 50% and 90% candidiasis, respectively. In terms of fluconazole, 128 μg/mL concentration was obtained as MFC and a concentration of 0.5 μg/mL as MIC50 (29).
In the study of Doddanna et al., which investigated the inhibition effect of the extract of the plant against Candida albicans, the results showed that the other plant extracts like alcoholic onion leaves, alcoholic tea leaves, alcoholic onion bulb, alcoholic Aloevera, and alcoholic mint leaves also inhibit the growth of Candida albicans but lesser than the extent (30).
In Prabhakar et al., which isolated 46 species of Candida albicans, antifungal activity of ethanol extracts of five plant species that included Syzygium jambolanum, Cassia siamea, Odina wodier, Momordica charantia, and Melia azedarach and two algal species, Sargassum wightii and Caulerpa scalpelliformis were tested against 25 isolated strains by disc diffusion method. Antifungal activity was observed at 100 mg/mL for Syzygium jambolanum, Cassia siamea, and Caulerpa scalpelliformis, and at 10 mg/mL for Sargassum wightii (31).
In another study, antifungal activity of Mentha piperita/Mentha Arvensis, Aloe vera, Murraya koenigii, Allium cepa, Camellia sinensis were investigated. The results showed that the raw extract of tea leaf had the highest inhibition zone against Candida albican (30). In the study of Masom et al., which included antifungal activity of 5 plant species Trachyspermum ammi (seed), Teucrium polium (leaf), Piper nigrum (seed), Pistachia vera (skin), and Camelia sinensis (leaf), the results showed that all plant extracts inhibited Candida albicans, while the largest inhibition zone was for Pistachio vera (40 mm).
The lowest antifungal activity was produced by Piper nigrum (13 mm) and the minimum inhibitory concentration and the minimum inhibitory concentration of Pistachio vera were 6.25 and 12.5 mg/mL (32), respectively.
5.1. Conclusions
In general, since various studies have confirmed the antimicrobial, anti-cancer, and antioxidant properties of rosemary essential oil (33).
The results of this study showed that methanolic extract of rosemary at different concentrations and times has antifungal and antiparasitic effects that can be used to treat infections caused by them.