1. Context
The use of herbal medicinal compounds has long been considered by a human being. The drugs that are used widely worldwide to cure several diseases, including bacterial, viral, and fungal infections, and all kinds of metabolic diseases, even cancer, are naturally occurring compounds (1-3). The herbal medicines containing herbal compounds and their derivatives account for about one-third of the total available drugs (4, 5). One of the main reasons for the desire of the medical community to use herbal compounds is their low side effect compared with chemical drugs, which has been proved during many years in traditional medicine (6, 7). In addition, the use of industrial medicines and chemical compounds can lead to undesirable metabolic reactions, and, in most cases, free radicals and peroxides are produced. Herbal medicines are rich in various bioactive compounds that prevent the production of oxidants (8).
Enterohemorrhagic Escherichia coli is one of six types of E. coli recognized as the cause of diarrhea. It provides cytotoxins, such as verocytotoxin or Shiga-like toxin, which causes hemorrhagic colitis. This organism was first identified as a cause of illness in 1982, and its related diseases have been increased. E. coli has recently been widely considered as it is easily transferred by human and animal waste and contaminates the environment (9, 10).
E. coli is an opportunistic pathogen that is frequently colonized in the human colonic region (11). At the equal opportunity, it is one of the most prevalent causative agents for the urinary tract injury (UTI) and abdominal diseases. The most important virulence part of microorganisms, especially Gram-negative bacteria, is adhesion to the host cell receptors. This adhesion is caused by particular adhesins, which are surrounded in fimbriae, e.g., pili (pilus adhesion) in Gram-negative bacteria and are on the cell wall (non-pilus adhesion) in Gram-positive bacteria.
E. coli can acquire antimicrobial resistance mechanisms, such as encoding the genes of the enzymes, such as β-lactamases, the genes that alter the bacterial cell wall, which results in no binding site for antimicrobial agent and condensation of outlet pumps, translation, and transduction (12). Efflux pumps are transportation proteins involved in the extrusion of toxic substrates (including nearly all types of clinically important antibiotics) (13). Efflux pumps can be discriminated from multidrug efflux pumps, and the extrusion of antimicrobial agents via these efflux pumps is a major factor in antimicrobial resistance (14). Cells can use proton-driven antiporters and/or ATP-driven transporters (ATP-binding cassette) to expel medicines (15). Efflux pump inhibitors can be utilized to reduce the efflux of antibiotics from bacterial cells, suggesting that antibiotics, such as ciprofloxacin can be used to restore medicinal protection. Verapamil and MC-207,110 are common utilized efflux pump inhibitors (15). As a consequence of antibiotic defense, new antimicrobials that will be restored or used with antibiotics are needed.
2. Evidence Acquisition
Studies in English published from 2000 to 2017 were searched using Web of Science, PubMed, Scopus, Google Scholar, and ScienceDirect using the keywords Escherichia coli, Plant extracts, herbal plants, herbal medicines, and antibacterial activity. Further citations were recognized by evaluating the reference lists of related articles.
3. Results
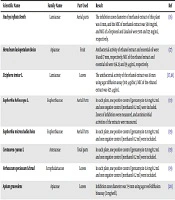
The reported minimum inhibitory concentration (MIC) values of the used plants against E. coli are summarized in Table 1. As can be found, the majority of the studied plants had bacteriostatic activity, and some had both bacteriostatic and as well as bactericidal activity.
| Scientific Name | Family Name | Part Used | Result | Ref |
|---|---|---|---|---|
| Stachys inflata Benth | Lamiaceae | Aerial parts | The inhibition zone diameter of methanol extract of this plant was 11 mm, and the MIC of methanol extract was 500 mg/ml, and MIC of a-Terpineol and Linalool were 500 and 125 mg/mL, respectively. | (16) |
| Heracleum lasiopetalum Boiss | Apiaceae | Fruit | Antibacterial activity of ethanol extract and essential oil were 18 and 17 mm, respectively. MIC of the ethanol extract and essential oil were 156.25 and 39 µg/mL, respectively. | (17) |
| Ziziphora teniur L. | Lamiaceae | Leaves | The antibacterial activity of the ethanol extract was 18 mm using agar diffusion assay (100 µg/disc). MIC of the ethanol extract was 625 μg/mL. | (17, 18) |
| Euphorbia helioscopa L. | Euphorbiaceae | Aerial Parts | In each plate, one positive control (gentamycin 0.8 mg/0.2 mL and one negative control (methanol 0.2 mL) were included. Zones of inhibition were measured, and antimicrobial activities of the extracts were measured. | (19) |
| Euphorbia microsciadia Boiss | Euphorbiaceae | Aerial Parts | In each plate, one positive control (gentamycin 0.8 mg/0.2 mL and one negative control (methanol 0.2 ml) were included. | (19) |
| Centaurea cyanus L | Asteraceae | Total parts | In each plate, one positive control (gentamycin 0.8 mg/0.2 mL and one negative control (methanol 0.2 ml) were included. | (19) |
| Verbascum speciosum Schrad | Scrophulariaceae | Leaves | In each plate, one positive control (gentamycin 0.8 mg/0.2 mL and one negative control (methanol 0.2 ml) were included. | (19) |
| Apium graveolens | Apiaceae | Leaves | Inhibition zone diameter was 7-9 mm using agar well-diffusion bioassay (2 mg/well). | (20) |
| Trigonella foenum-graecum | Leguminosaepapilionoideae | Seeds | Inhibition zone diameter was >15mm using agar well-diffusion bioassay (2 mg/well). | (20) |
| Ziziphus ziziphus | Rhamnaceae | Fruit | Inhibition zone diameter was 10-14 mm using agar well diffusion bioassay (2 mg/well). | (18, 20, 21) |
| Rhus coriaria | Anacardiaceae | Fruit | Antibacterial activity of the ethanolic extracts using the disc and well diffusion assays was 17 and 24 mm, respectively. The zone of inhibition was 17 mm through disc diffusion assay. MIC of extracts was 0.20%. | (22) |
| Funmaria vaillantii | Fumariaceae | Flowers and stems | The zone of inhibition using the agar diffusion test was 11 mm and MIC was 125 µg/mL. | (23) |
| Quercus brantii | Fagaceae | Fruit | The inhibition zone diameter was 12 mm. | (24) |
| Artemisia siberi | Asteraceae | Aerial parts | The diameter of the inhibitory zone diameter was 12 mm. | (25) |
| Wasabia japonica | Brassicaceae | Total part | The results indicated that the MIC of wasabi against E. coli O157:H7 and S. aureus was 1% (equal to 10 mg/mL) and 4%, respectively. | (26) |
| Peganumharmala | Nitrariaceae | Fruit | MIC was 5 mg/mL. | (27) |
| Peganumharmala | Nitrariaceae | Root | MIC was 0.625 mg/mL. | (27, 28) |
| Allium sativum | Amaryllidaceae | Fruit | The inhibition zone diameter around the discs varied from 17 - 35 mm, indicating that all 13 AmpC positive isolates (100%) were sensitive to garlic extract. One out of 13 E. coli sequesters had a MIC of 2.5 mg/ml for alcoholic distillate of A. sativum. The highest MIC and MBC values of the alcoholic distillate of A. sativum were 5 mg/ml and 10 mg/mL, respectively. The highest and lowest MIC of AmpC positive E. coli isolates were determined to be > 256 and 16 µg/mL, respectively. | (29) |
| Trachyspermum ammi | Apiaceae | Seed | The highest MIC of the needful oil was 100 ppm against E. coli. | (18, 30, 31) |
| Hibiscus sabdariffal | Malvaceae | Flower | The highest MIC value was detected to be 20 mg/mL against two E. coli isolates. | (32) |
| Cassia auriculata | Fabaceae | Leaves | The MIC and MBC of ethyl acetate extract of the leaves and flowers were measured. The highest MIC was 50 - 125 mg/mL. | (33) |
| Artichoke | Asteraceae | Leaves | The results showed that the inhibition zone diameter of the ethanolic, methanolic, and estrogenic extracts of leaves was 11 mm, while for the ethanolic and methanol extracts of the stem, this rate was equal to 8 - 14 and 11 mm, respectively | (34) |
| Punica granatum | Lythraceae | Peel | The PIC of the ethanol extract prepared from peel was 14.2 ± 0.61 | (35) |
| Zataria multiflora | Lamiaceae | Leaves | The correlation coefficient of the concentration of essential oil of Z. multiflora with the logarithm of the bacteria was studied at temperatures of 4, and 10°C and the results were 0.701 and 0.599, indicating that by increasing the essential oil concentration, the growth rate of the bacteria during the storage period is reduced and efficacy of the necessary oil on the growth of bacteria is significant. | (36) |
| Honey | The results showed that the inhibitory zone diameter of honey, licorice, honey, apples, and honey are 1 ± 13, 5/0 ± 12, and 5/0 ± 9 mm | (37) | ||
| Rose | Rosales | Leaves | The inhibitory zone diameter of the ethanolic extract of rose was 14 mm, and its MIC was 25 mg. | (38, 39) |
| Aloe vera | Asphodelaceae | Leaves | MIC was 50 µg. | (39) |
| Fennel Bakhtiari | - | - | MIC and MBC of alcoholic extract of Bakhtiari fennel against E. coli were 3.12 mg/ml and zero, respectively. | (40) |
| Peganum harmala | Nitrariaceae | Leaves | Compared with the controls, there was no significant difference n the efficacy of the P. harmala juice on microbial growth. | (41, 42) |
| Eucalyptus globulus | Myrtaceae | Leaves | The results indicated that essential oil of the leaves of E. globulus has an antibacterial effect against E. coli and S. aureus. | (41, 42) |
| Cyminum cuminum | Apiaceae | Seed | Ethanol extract of seeds showed antimicrobial activity against the E. coli biofilm. | (31, 43) |
| Zataria multiflora | Lamiaceae | Leaves | The extract represented inhibitory activity against E. coli. | (44) |
| Coccinia grandis | Cucurbitaceae | Leaves | Aqueous, acetone, and ethanol extracts of the leaves of C. grandis were examined for antibacterial effect using the agar well diffusion method. Ethanol extract of the leaves displayed antibacterial effect against biofilm producing strains UPEC 17 and 82, whereas the aqueous and acetone extracts displayed antimicrobial effect only against UPEC 57. Ethanol extract of levees showed inhibitory action against ESBL producing UPEC 87 and 96, while the aqueous extract inhibited the growth of only UPEC 85. | (45, 46) |
| Avicenna Marina | Acanthaceae | - | The glycerol extract of Avicenna Marina showed inhibitory activity against E. coli and P. digitatum. | (45, 46) |
| Calotropis procera | Apocynaceae | Leave | The results illustrated that a total of 30 of 80 (37.5%) isolates harbored ESBL enzymes. | (47) |
| Punica granatum | Lythraceae | Leave | MIC of Punica granatum was 4.0mg/ml, which showed a good antibacterial activity against Shiga toxin produced by E. coli. | (48) |
| Punica granatum | Lythraceae | Leave | MIC was 0.49 to 1.95 mg/ml and MBC was 1.95 to 3.91 mg/ml. | (49) |
| Punica granatum | Pomegranate | Leave | In this study, six extracts were prepared from the powdered leaves of P. granatum. The MIC was found to be from 0.5 to 20.07%. The highest MIC was obtained using the aqueous extract and the lowest was related to the ethyl acetate extract. | (50) |
| Ocimum gratissimum | Lamiaceae | The MIC presented by Ocimum gratissimum against the E. coli strains was 20,000 μg/mL. | (51) | |
| Butea monosperma | Fabaceae | Flower | The extract exhibited antibacterial activity against E. coli and S. aureus. | (52) |
| Physalis pubescens | Solanaceae | Leaves | The extracts exhibited the highest inhibitory diameter zone of 12.5 to 13.6 mm. | (53) |
| Ruellia tuberosa L. | Acanthaceae | Root | The inhibitory diameter zone against E. coli, was 7, 7, 7, 10.75, 11.00, and 15.00 mm for the concentrations of 5, 10, 20, 50, 75, and 100% (v/v), respectively. | (54) |
| Rubia cordifolia | Rubiaceae | Root | The plant could be a potential candidate for an alternative antibacterial agent to combat the invasion of drug-resistant organisms. | (55) |
Abbreviations: E. coli: Escherichia coli; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration.
4. Discussion
Several studies have revealed that herbal medicines are good sources of compounds with antioxidant and antimicrobial activity, which are able to protect the body against cellular oxidation and pathogens. Therefore, the classification of various herbal medicines because of their antioxidant and antimicrobial potentials is considerable. Herbal drugs that are safe and protect against pathogens are beneficial candidates for generating new antimicrobial medicines and have been long used in many cultures.
The main medicinal plants and their major compounds that can affect E. coli are as follows: Allicin (S-(2-propenyl) 2-propene-1-sulfinothioate) is the most biologically active sulfur-containing compound of garlic (A. sativum) (56), thymol, γ-terpinene, para-cymene, and α- and β-pinene are the most biologically active sulfur-containing compounds of Trachyspermum ammi), the major compounds identified in the essential oil of Hibiscus sabdariffal are hexadecanoic acid and linoleic acid; Carvacrol and Thymol are the most frequent compounds of the Zataria multiflora Z (57); linalool and β-pinene are the most biologically active sulfur-containing compound of Aloe vera and Teucrium polium (58); in Eucalyptus globulus, 1,8-cineole, globulol, trans-pinocarveol, and alpha-terpineol are the main components (59); Cuminaldehyde, p-cymene, β-pinene, α-terpinen-7-al, γ-terpinene, p-cymen-7-ol, and thymol are found in Cyminum cuminum (60), n-tetracosane, n-eicosane, tetratriacotane, 7-octadecanal , and tricosane are the major constituents in Coccinia grandis (61), (the major compounds in the leaf essential oil of Laportia ovalifolia and Spondias mombin are δ-cadinene, α-humulene, γ-muurolene, α-gurjunene, α-muurolene, 5-isocedranol, and δ-cadinene) (62), in Ficus exasperate, the significant compounds are 1,8-cineole, (Ε)-phytol, and p-cymene) (63), and sesquiterpene β-caryophyllene (flower: 15.2%, stem: 8.1%) are highly found in Ageratum conyzoides; six of the identified compounds, including β-copaene, hexanal, trans-cadina-1(6),4-diene, α-calacorene, caryophylla-4(12),8(13)-diene-5-β-ol, and 1,10-di-epi-cubenol are reported for the first time as constituents of A. conyzoides) (64); the main chemical constituents in Punica granatum peel seeds were propanoic acid, benzenedicarboxylic acid, methoxypropionic acid, and methylamine (65), regarding Quercus Infectoria, both phenolic and flavonoid compounds are found in the methanol extract; however, phenolic compounds are more than the flavonoid compounds (66); phenolic compounds, flavonoids, saponins, steroids, tannins, xanthoproteins, carboxylic acids, coumarins, and carbohydrates were detected in the methanol extract of Peltophorum pterocarpum flowers (67); in P. granatum, Cissus welwitschii, Triumfetta welwitschii, Entada Africana (bark), Lannae acida (stem bark), and Terminalia avicennoides, bioactive hydrolysable tannin compounds, including ellagic acid, punicalagin, flavogallonic acid, and terchebulin) (68) are highly available; four flavonoids, such as 6,7-(2”,2”-dimethyl chromeno)-8-γ,γ-dimethyl allyl flavanone, 3’,4’dihydroxy-7,8 (2”,2”- dimethyl chromeno)-6-γ,γ dimethyl allyl flavonol, 7-methyltectorigenin, and Irisolidone were isolated from the leaves of Lannea acida) (69); α-pinene, E-β-ocimene, terpinolene, α-terpineol, eugenol, β-cubenene, β-caryophyllene, γ- muurolene, -muurolene, epi-cubebol, and -cubebol, and δ-cadinene in less quantity were detected in Ocimum gratissimum (70); and new anthraquinones, namely 1-hydroxy-2,7-dimethyl anthraquinone, 2-hydroxy -6-methyl anthraquinone, 2,6-dihydroxy anthraquinone, 1-hydroxy 2-methyl anthraquinone, nordamnacanthal, physcion, 1,4-dihydroxy 6-methyl-anthraquinone, 1,4-dihydroxy 2-methyl anthraquinone, 1,5-dihydroxy 2-methyl anthraquinone, 3-prenyl methoxy 1,4-naphthoquinone, 1-hydroxy 2-methoxy anthraquinone, 1,4-dihydroxy 2-methyl 5-methoxy anthraquinone or 1,4-dihydroxy 2-methyl 8-methoxy anthraquinone, 1,3-dimethoxy 2-carboxy anthraquinone, and rubiadin were isolated from Rubia cordifolia roots) (71). Additional information about medicinal plants effective against E. coli are presented in Table 1.
5. Conclusions
The strains of E. coli have consistently been shown resistance to various antibiotics, which has increased their pathogenicity; thus, finding new strategies to treat infections caused by them is of great importance.