1. Background
Cerebral ischemia is one of the most harmful and hazardous ailments in the world and one of the leading causes of deaths in the developed and developing countries, the mysterious nature of which has not yet been fully discovered (1). Ischemia is one of the strongest stimuli for apoptotic gene expression in the brain (2). Apoptosis, or programmed cell death, is a set of cellular events that lead to cell damage and its contents, and Bcl-2 and Bax are the key elements of apoptosis that are strongly expressed and regulated in the brain. Bcl-2 is an anti-apoptotic membrane protein, and Bax is a pro-apoptotic protein (3). Physical activity and exercise can now be considered a neuroprotective agent (4). Physical activity increases the expression of neurotrophic proteins (5). Neurotrophins are small proteins that bind to tyrosine kinase (Trk) receptors and perform various functions in neurons (1). Binding of these neurotrophins to the TrkB receptor activates many signaling-dependent molecules, including neurogenesis, synaptic density, and neuronal plasticity (1, 4). A number of studies have suggested that alterations in the signaling pathway of the TrkB pathway are essential for affecting exercise on the brain and can modulate synaptic plasticity and translocation because blocking this signaling pathway may inhibit memory and exercise-induced learning (6-9). Exercise is also thought to be the underlying mechanism of suppression of apoptosis by TrkB (10). Despite extensive research into the effects of exercise on neurodegenerative diseases, especially brain tissue-related injuries, it appears that many questions for underlying mechanisms of them remain to be clarified. Most of these studies have specifically focused on neurotrophic changes and their underlying cell-molecular mechanisms, including gene expression that can occur by changes in the receptor, needing further investigation. Since gene expression is a process in which the information within the gene is used to obtain a functional product, it is considered one of the substrates of evolution because it allows the genotype to appear as a phenotype. This allows the cell to control its structure and function, cellular differentiation, evolution, and the ability of organisms to adapt to new conditions.
2. Objectives
Therefore, with the knowledge gained so far and discovering new ideas and ways to support brain health and function, this study aimed to compare two types of training programs on TrkB, Bax, Bcl-2 gene expression in male Wistar rats after the induction of cerebral ischemia.
3. Methods
3.1. Animals
In this study, 40 adult male Wistar rats (mean age: 40 - 45 weeks, weight: 250 - 300 g) were used. All experiments were performed in accordance with the Ethics Committee of the Bojnourd University of Medical Sciences.
3.2. Study Groups
Animals were randomly divided into four groups of ten subjects, as follows:
3.2.1. Endurance Training
In this group, rats were first swimming in rodent pool for three weeks and five days each week for 30 minutes each day, and cerebral ischemia was induced after a period of training (11).
3.2.2. Endurance-Cognitive Training
The training included swimming in the Morris Water Maze. Each rat trained for three weeks, three days per week, four times each day for 10 min. Cerebral ischemia was induced after the training period (12).
3.2.3. Ischemia
In this group, the rats were kept in the same condition for three weeks, and in the end, cerebral ischemia was induced.
3.2.4. Control
The rats were kept in the same condition for three weeks and were not induced by ischemia.
3.3. Induction Cerebral Ischemia
To induce cerebral ischemia, the rats were anesthetized with ketamine/xylazine (30 mg/kg, IP). Then, under sterile conditions, both common carotid arteries were occluded for 30 min using Yashargil Aneurism clips. Next, the carotid arteries were released and inspected for immediate reperfusion (13).
3.4. Preparation of Specimens for Real-Time PCR
Twenty-four hours after ischemia, the rats were anesthetized using chloroform, and after the heart blood sampling, their skulls were cleaved to extract the brain. To measure the expression level of TrkB, Bcl-2, and Bax genes in the brain tissue, Real-time PCR (polymerase chain reaction) method was used. At the beginning of the assay, the brain tissue was crushed and lysed. RNA extraction was performed using RNX-Plus SinaClon, Iran solution according to manufacturer’s instruction. RNA quality was first assessed by electrophoresis with 1% agarose gel, and then the purity of the extracted RNAs was determined by Nanodrop device, and the optical absorption ratio of 260 nm to 280 nm was measured. In this study, samples with an optical absorption ratio of 1.8 to 2 were used for cDNA synthesis. The cDNA was synthesized by Yekta Tajhiz Azma-Iran cDNA synthesis kit. Thus, one microliter of oligo dT primer was added to one microgram of RNA, and finally, the volume of the solution reached 13.4 microliters by DEPS water, and the solution was placed at 70ºC for 5 minutes. After that time, it was immediately put on ice. In the second step, a mixture of four microliters of five standard buffer, half microliter of RNasin, one microliter of dNTPs, one microliter of M-MLV was prepared and added to the vial containing RNA. The PCR reaction was performed based on a 60 min cycle at 42ºC and at the end of five minutes at 70ºC. The obtained cDNA was then stored at -20ºC. In this study, real-time PCR Illuminar was used by SYBR Green method of Yekta TajhizAzma Co.-Iran to determine the expression of the target genes. Materials for performing Real-time PCR in a volume of 20 µL consisted of 10 µL of SYBR Green, 7.6 µL of water, 0.7 µL of forward-reverse primers and 0.5 µL of cDNA template. The primers required for the reaction were designed by Primer 3 software. The sequences of TrkB, Bcl-2, and Bax primers as the target genes and GAPDH as the house-keeping are shown in Table 1. The PCR conditions were 10 minutes at 95ºC (initial denaturation), 10 seconds at 95ºC (denaturation), 15 seconds at 56ºC (primer binding), 15 seconds at 72ºC (expansion). After completion of each PCR reaction, electrophoresis was performed on 1.5 agarose gel to evaluate the quality of PCR product replication. Standard curves for TrkB, Bcl-2, Bax, and GAPDH genes were plotted before Real-time PCR was performed on the samples. Binding efficiency of primers to different genes was measured using standard curve plots. The standard curve was depicted based on the logarithm of concentration and threshold cycle (CT) for each gene. Data analysis was performed using CT comparison. Data were analyzed by 2-ΔΔCT method according to Livak method (14). The expression levels of TrkB, Bcl-2, and Bax genes were measured in comparison to GAPDH gene as the internal control.
| Primer sequence (5'->3') | Product Size |
|---|---|
| Ntrk2 | 128 |
| F: CACTCTTGGCGTAGATTGGC | |
| R: AAGTAAACTCTCGGGGTGGG | |
| Bcl2 | 136 |
| F: CACATCTCAGTTCCCTTGGC | |
| R: TCTTCTCCCTCTAGCACACC | |
| Bax | 141 |
| F: GCTACAGGGTTTCATCCAGG | |
| R: TTGTTGTCCAGTTCATCGCC | |
| GAPDH | 127 |
| F: CAACTTTGGCATCGTGGAAGG | |
| R: AGGGATGATGTTCTGGGCTG |
The Sequences and Product Size of Forward and Reverse Primers
3.5. Statistical Analysis
The Shapiro-Wilk test was used to examine the normality of data distribution. One-way ANOVA and Tukey’s post hoc test were used to analyze the data by SPSS (Chicago, IL, USA; Version 22) software (P < 0.05).
4. Results
4.1. Findings on TrkB, Bcl-2 and Bax Variables
Given the presence of parametric statistics assumptions, findings on TrkB, Bcl-2, and Bax genes were analyzed in the groups using one-way analysis of variance (Table 2).
| Sum of Squares | df | Mean of Squares | F | Sig. | |
|---|---|---|---|---|---|
| TrkB | |||||
| Between-group | 167.05 | 3 | 55.68 | 26.45 | 0.001* |
| Within-group | 75.76 | 36 | 2.10 | ||
| Total amount | 242.84 | 39 | |||
| Bcl-2 | |||||
| Between-group | 105.25 | 3 | 35.08 | 9.13 | 0.001* |
| Within-group | 138.32 | 36 | 3.84 | ||
| Total amount | 243.57 | 39 | |||
| Bax | |||||
| Between-group | 44.69 | 3 | 14.89 | 3.39 | 0.028* |
| Within-group | 158.06 | 36 | 4.39 | ||
| Total amount | 202.74 | 39 |
The Results of the Analysis of Variance (ANOVA) on TrkB, Bcl-2, and Bax Genes Expression
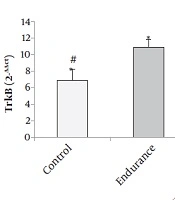
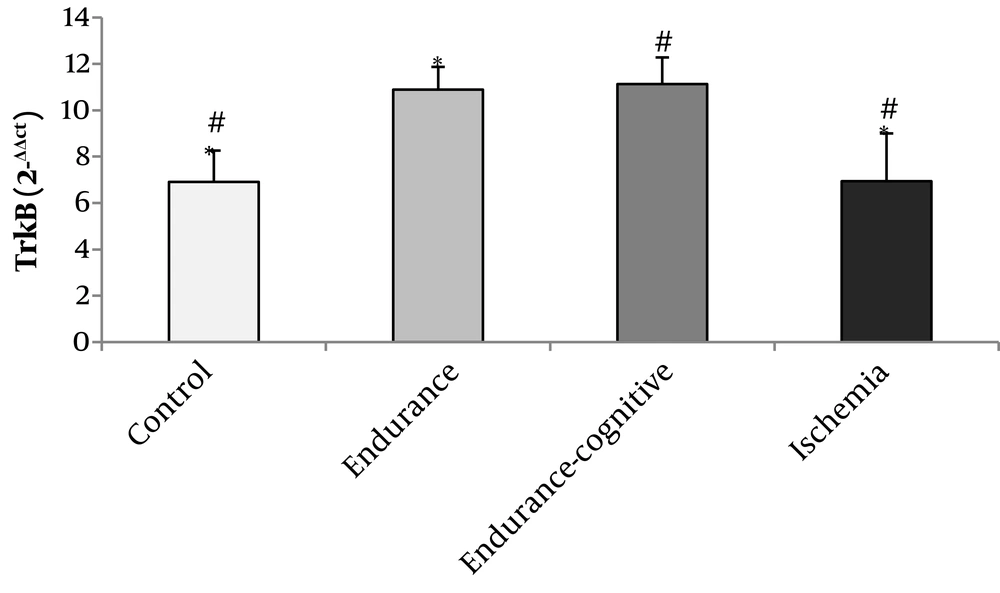
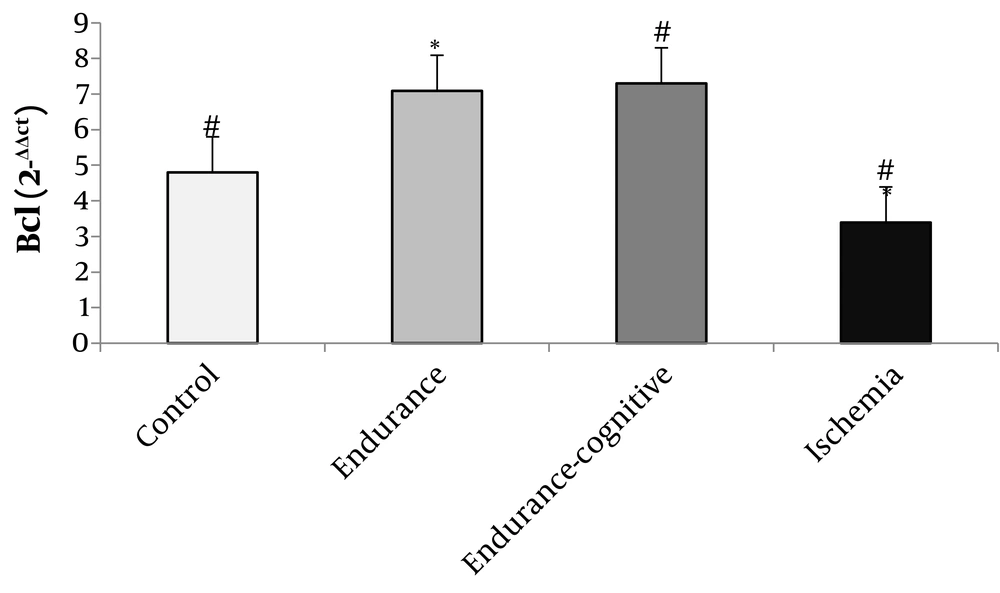
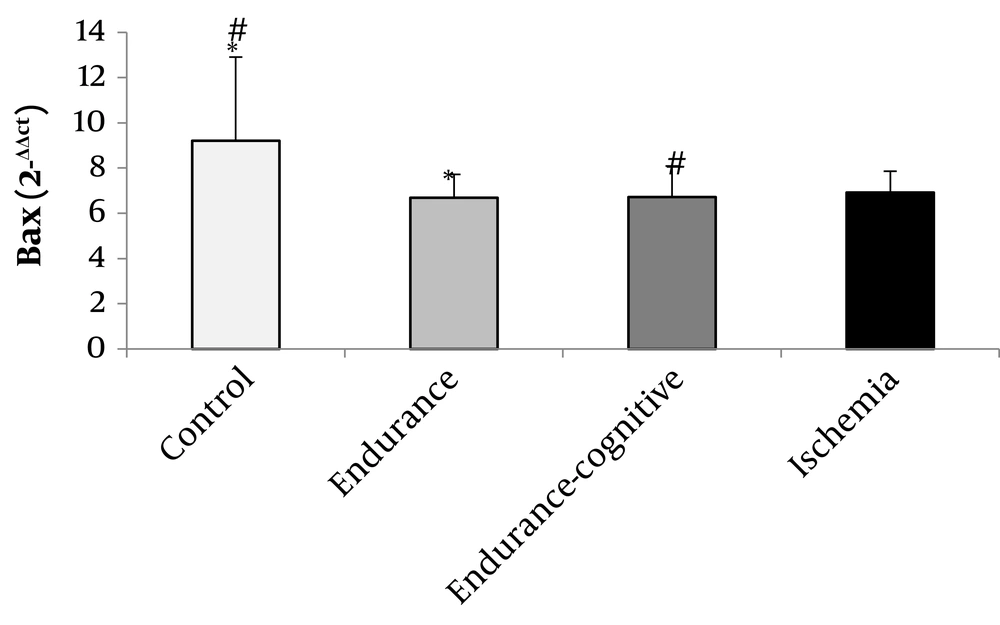
The results of Figure 1 show that the levels of TrkB gene expression significantly increased in the endurance training and endurance-cognitive groups compared to the ischemia and control groups (P < 0.05). The results of Figure 2 show that the levels of Bcl-2 gene expression increased in the endurance training group compared with the ischemia group as well as the endurance-cognitive group compared with the ischemia and control groups (P < 0.05). The results in Figure 3 show that the levels of Bax gene expression significantly decreased in the endurance training and endurance-cognitive groups compared to the control group.
5. Discussion
Many studies report that physical activity of any type and intensity causes slight but constructive changes in the brain (1). Zhang et al. in a review article entitled “the effects of physical activity on stroke” stated that rats, which experienced a short period of stroke before exercise had a superior motor function, more active cells in the pre-infarction area, a decrease in infarct size, and an increase in angiogenesis. Therefore, a short period of pre-stroke physical activity may be associated with enhanced brain tissue (1). The results of the present study showed that both training models could significantly increase TrkB gene expression. Consistent with the present results, Allen et al. by studying eight weeks of treadmill running for 5 days a week for 30 minutes daily, showed that TrkB gene expression was significantly increased in the training group compared to the control group. These investigators using the ANA-12 antagonist, which blocks TrkB-dependent signaling pathways, have shown that protective effects of exercise on the nerves and cognition will not be exercised if the receptor fails to function (9). Lee et al. examined the effect of optional training, including running on cycling wheel and resistance training for four weeks on TrkB values in the hippocampus of male rats. The results showed that both of these training models improved cognitive performance. However, endurance training increased TrkB and CREB values more than aerobic training (15). Vedovelli suggested that binding of BDNF to TrkB and the p75 neurotrophin receptor activates biochemical cascades that can lead to cell proliferation, survival, and plasticity, and at least partially explain the effects of cognitive training conditions on the brain (11). However, the results of the study of Macias et al. in contrast to our results, showed that 28 days of walking on the treadmill increased BDNF mRNA levels but did not affect TrkB gene expression. The causes of this inconsistency include the measurement of these proteins in the lumbar spinal cells. The researchers suggest that the increase in BDNF has more affectability than its receptor and that the increase is not sufficient to increase the receptor (16). Nichol et al. showed that six-weeks of optional running on a circular wheel led to an increase in the TrkB receptor compared to the immobilized group, and this increase was associated with improved spatial memory in the animal (17).
Concerning the Bcl-2 and Bax variables, the results showed that the training could significantly increase Bcl-2 and decrease Bax. Ischemia-induced events increase oxidative stress, leading to cellular damage such as cell mitochondrial apoptosis. In many brain-related diseases, especially stroke increased apoptosis likely occurs, and its prominent signs are an increase in the number of Bax proteins and a decrease in Bcl-2 protein, leading to the intensification of apoptosis and ultimately neuronal death (3, 18). Although there is conflicting information about the effect of exercise training on apoptotic-dependent activities and most of these exercises were specific to running on the treadmill, it seems that exercise balances oxidation and resuscitation and improves brain function by increasing resistance to oxidative stress and accelerating recovery (10, 18). Consistent with the results of the present study, in a study by Zhang et al., it was shown that three weeks of exercise training on circular wheel could decrease Bax/Bcl-2 ratio. Exercise reduces oxidative stress and apoptosis and repairs DNA damage (10). Fang et al. also report that 12 weeks of treadmill training increases Bcl-2 levels. The researchers believe that exercise through phosphorylation of protein kinase B leads to a decrease in the levels of pro-apoptotic factors Bax and cytochrome C, thereby inhibiting apoptosis in the brain (19). On the other hand, Aboutaleb et al., in their study, examined the effect of exercise on Bax/Bcl-2 protein expression after the induction of cerebral ischemia. The results showed that in the ischemic group, Bax/Bcl-2 ratio was significantly increased. Researchers assert that exercise by reducing the Bax/Bcl-2 ratio reduces post-ischemic nerve damage. The mechanism of protective effects of exercise includes preventing the cytotoxicity of NMDA receptors and reducing ROS production. These neuroprotective mechanisms of exercise can provide treatment that can both elevate cell survival and decrease neuronal death (13). Santana et al. conducted a study on male Wistar rats following a 13-week aerobic training period on the treadmill and reported an increase in the expression of Bcl-2 gene and anti-apoptotic factors protein (20). Liebelt et al. regarded preconditioning with exercise as a factor in reducing apoptotic factors in post-stroke rats. The results showed that exercise reduced apoptosis and cerebral infarction (21). Hamakawa et al. showed that three weeks of pre-training along with exercise reduced oxidative stress markers and increased the activity of antioxidant enzymes, which was associated with reduced infarct volume and improved neurological functional outcomes after ischemic damage (22). Finally, it can be concluded that exercise activity in the present study was able to exert its protective effects by altering the expression of the target genes. As a result, exercise could significantly reduce apoptotic conditions and increase anti-apoptotic and neurogenic factors in the training groups indicating that such exercises are beneficial in cerebral ischemia. To further substantiate this assertion, further research is needed by examining other signaling pathways and other factors involved.
5.1. Limitations and Suggestions
Questions remain to be addressed in future studies, including the effects of different types of training with different intensity, such as the effect of resistance training on cerebral ischemia, the effect of exercise on other Trk receptors and neurotrophins on the cellular, and molecular basis of neurons in the brain tissue.