1. Background
One of the major challenges created by petroleum industries is hydrocarbons that contaminate soil (1, 2). Slurry sequencing batch reactors (S-SBR) are an ex-situ bioremediation method (3) that are used to treat oil contaminated soils (4). Bioremediation rate in an S-SBR is higher than in situ or solid-phase systems; also, it has greater mass transfer rates (3, 5), and is more manageable, controllable, and predictable (6). Robles-Gonzalez et al. proposed the bio-slurry reactor as a technology, which could be used for bioremediation of problematic sites (7).
The density of soil organisms is in the range of 104 to 107 CFU/gram of soil (8). For favorable degradation, this density should not be lower than 103 per gram of soil (9). Organism density lower than 103 CFU per gram of soil showed the probability of existence of toxic contaminants (10, 11).
Diesel oil is a complex compound (12) and contains n-dodecane and n-hexadecane (8). N-hexadecane is released to the environment from gas stations, rubber manufacturers, shale oil production, coal combustion, biomass and refuse combustion, and tobacco smoking (13, 14). N-dodecane is used as a solvent, distillation chaser, and scintillator component. It is used as a diluent for tributyl phosphate (TBP) in reprocessing plants. In the recent years, n-dodecane has garnered attention as a possible surrogate for kerosene-based fuels, such as Jet-A, S-8 and other conventional aviation fuels (15).
Due to different oil compounds, n-alkanes with middle length chain (C10 - C25) are the favored substrate for organisms and are the most quickly degradable (9). In addition, longer chain alkanes (C25 - C40) are hydrophobic solids and therefore are hard to degrade due to their poor water solubility and bioaccessibility (16). In addition, in some studies (8, 17), n-hexadecane and n-dodecane were chosen as the model pollutants for diesel oil. Thus, in the present study, n-alkanes with intermediate chain (n-hexadecane (C16H34) and n-dodecane (C12H26)) were selected.
In different studies, degradation of n-hexadecane and n-dodecane from the soil was studied by pure (8, 18) and consortium cultures, including degradation of n-hexadecane from the soil by pure and consortium cultures (19), aerobic degradation of n-hexadecane from soil by a pure culture (8), and the effect of co-metabolism on degradation of n-dodecane and n-hexadecane by a microbial consortium from the soil (20, 21).
Alkanes are important causes of soil contamination after oil spills. Isolation of microorganisms with the capacity of alkanes degradation is a cheap way for clean-up of the environment and solves problems related to oil industries. Iran is one of the biggest oil producers in the Persian Gulf region (22). In most areas, the contamination resulting from these products (gasoline, diesel and etc) is growing, and the contamination of soil and water resulting from these contaminants has been a problem. Thus, further studies are necessary to manage these contaminants.
2. Objectives
This study aimed at determining simultaneous bioremediation of n-hexadecane and n-dodecane from soil in an S-SBR by two types of bacterial consortium. Besides, it tried to compare the ability of two types of bacterial consortium in the bioremediation of oil hydrocarbons. Also, it attempted to determine variations of operating parameters, such as dissolved oxygen, temperature, pH, and the number of active bacteria, during the bioreactor operating cycle.
3. Methods
In this study, all chemicals were purchased from the Merck Company (analytical grade, Germany).
The soil samples were collected at a depth of 30 cm from agricultural soil of Shiraz, Fars province, Iran (Table 1). Preparing the soil was continued by the following steps: (i) The soil was sifted with a 10-mesh (2 mm) strainer for screening the soil and reaching conformity. (ii) The soil was submerged in distilled water and autoclaved for 15 minutes at 121°C. (iii) To sterilize, dry and reach a fixed weight, the soil was situated in the oven at 160°C. (iv) The soil was sifted again with a 10-mesh (2 mm) strainer (23). (v) The soil was transmitted to a 1-L container. (vi) The soil was artificially contaminated with 1% concentration of n-dodecane and n-hexadecane. (vii) To the homogeneous dispersion of oil hydrocarbons, the soil was soaked in the solution (hexane, n-dodecane, and n-hexadecane solution) and regularly mixed at short intervals. (viii) To increase adsorption of oil hydrocarbons by the soil, the soil was placed at room temperature (25°C) for a one-week period (8, 20, 21).
| Properties | Amounts |
|---|---|
| N2 (%) | 0.09 |
| Cu (ppm) | 11 |
| Zn (ppm) | 12 |
| K (ppm) | 186 |
| Lime (%) | 44.85 |
| EC (MS/cm) | 1.21 |
| Clay (%) | 20 |
| Silt (%) | 46.4 |
| Sand (%) | 33.6 |
| Soil Texture | Loam |
| Mn (ppm) | 20.4 |
| Fe (ppm) | 10.2 |
| P (ppm) | 10.1 |
| Organic Carbon (%) | 0.93 |
| Humidity (%) | 50 |
| pH | 7.58 |
For effective growth, microorganisms must have a supply of nutrients (24). Tap water was used to provide the nutrients. The essential nutrients composed of the following mineral salts: NH4Cl (2.5 g/L), NaCl (0.5 g/L), MgSO4 (0.3 g/L), FeCl3.6H2O (0.3 g/L), CaCl2 (0.01 g/L), and MnCl2.4H2O (0.01 g/L) added to tap water. Afterwards, the pH of the solution was adjusted to about seven. Then, the solution was autoclaved at 121°C for about 15 minutes. Finally, the solution was added to the bioreactor (8, 20, 21).
For keeping the used bacteria in fresh form in the bioreactor, they were cultured weekly in mineral medium. To prepare the solid mineral medium, 1 g/L of yeast extract (for better growth of bacteria) and 15 g of agar medium was added to the jar. The solution pH was adjusted to about seven. Then, the solution was added to the plates. For preparing the supply of carbon, 20 μL of n-dodecane and n-hexadecane was poured on the plates uniformly. Finally, the plates were placed in the incubator at 37.5°C for 24 hours for bacterial growth (8, 20, 21).
In this study, bacterial consortiums type A (Acinetobacter radioresistence, Bacillus subtilis and Pseudomonas aeruginosa) and type B (Ochrobactrum oryzae, Bacillus sp. and Sphingomonas yanoikuyae) was used. The bacterial consortium was isolated from oil contaminated soil and compost (8). For increasing the number of bacterial consortium, previously cultured bacteria in plates were re-cultured in nutrient broth medium. For bacterial growth, the solution was placed on the mixer in the incubator at 37.5°C for 24 hours. Then, the solution containing the bacteria was transferred to the test tubes. To isolate bacteria, the test tubes were centrifuged at 4000 rpm for five minutes. To become certain of the homogeneous distribution of bacteria in the bioreactor, the optical density (OD) of the bacteria was adjusted at wavelength of 600 nm (OD = 1). Finally, isolated bacteria were added to the bioreactor (2, 20, 21).
To measure the number of active bacteria in the bioreactor, the samples were taken from the bioreactor at various times. Then, the samples were cultured at three dilutions (10-1, 10-2, and 10-3) on the nutrient agar medium. Next, considering the temperature of the bioreactor, the samples were placed in the incubator at various temperatures for 24 hours. After ensuring the growth of the bacteria, the colonies were counted by the colony counter. Finally, the number of active bacteria was reported based on CFU/mL (20, 21, 25).
To analyze the residual of n-dodecane and n-hexadecane, hydrocarbons were extracted from the soil through US.EPA method 355°C. First, the samples were taken from deposited sediments after the sedimentation phase and dried at 37.5°C. To remove moisture from the soil, 0.5 gram of the dried soil was combined with 0.5 gram anhydrous Na2SO4 (9). Four milliliters of hexane (as solvent) was added to the soil and mixed. The mixture was placed in an ultrasonic bath at 30°C for two minutes. Eventually, the upper portion of liquid of the container was transferred to test tubes. For better extraction, this process was repeated twice. Then, the test tubes were centrifuged at 4000 rpm for five minutes to isolate the soil and the upper portion of the liquid. Then, 1 mL of the surface liquid was sampled. Finally, 2 μL of the sample was injected to GC-FID (20, 21).
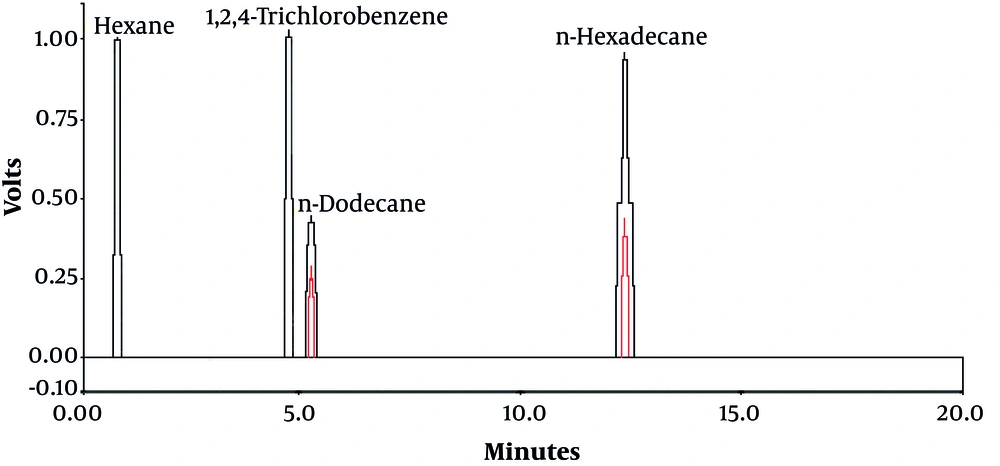
1, 2, 4-trichlorobenzene was used as an internal standard. CP-SILSCB (silica, USA) column (30 m length × 0.025 mm id × 0.25 µm film thickness) was used at a temperature program of 80°C for one minute, then increased to 125°C at 10°C/minute, held at 125°C for five minutes, then increased to 270°C at 40°C/minute, and held at 270°C for four minutes. The carrier gas was nitrogen and it was used at a constant flow rate of 2.7 mL/minute. The injector and detector temperatures were 210°C and 250°C, respectively. Also, the detection limit of the GC-FID for n-dodecane and n-hexadecane was 132 and 166.5 mg/Kg dry weight soil, respectively (2). The chromatogram of n-dodecane and n-hexadecane is presented in Figure 1.
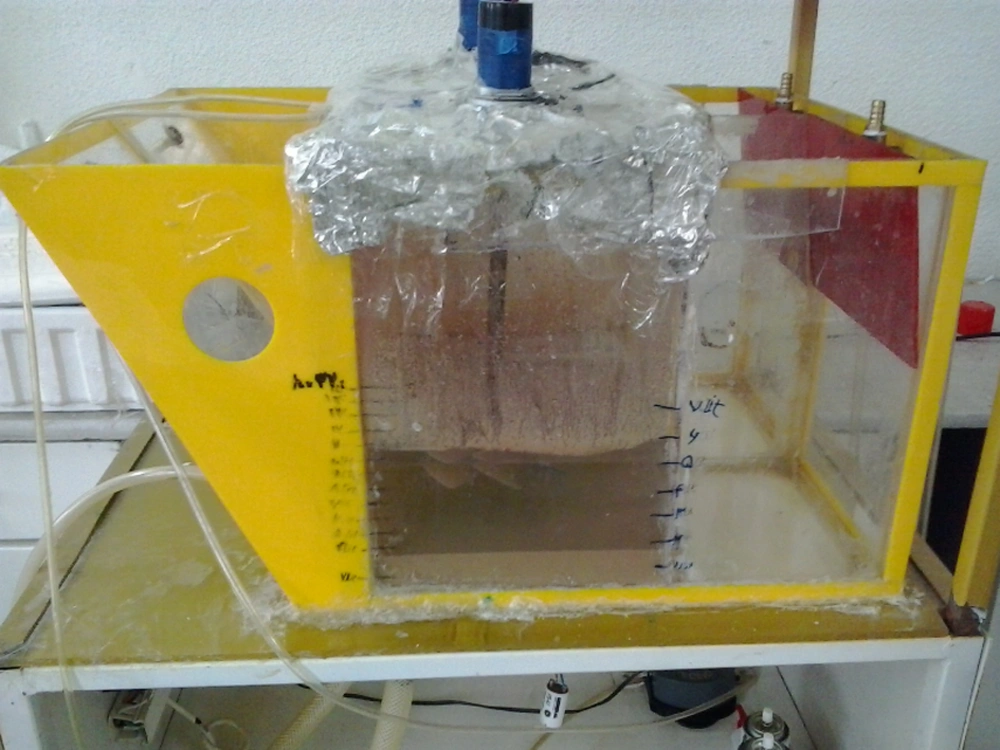
In a glass bioreactor (total volume = 11 L and working volume = 5 L), 250 g of oil hydrocarbons contaminated soil, the essential nutrients, and bacterial inoculation liquid was mixed with enough tap water (Figure 2). The samples were taken from both deposited sediments and upper liquid. In each cycle (74 hours), samples (8 mL) were collected. The first sample (T0) was taken one hour after start-up of the bioreactor (for being certain about the homogeneous distribution of the materials). Since the vapor pressures of n-dodecane and n-hexadecane were 20 Pa at 40°C and 100 Pa at 105.3°C, respectively (26), sediment samples were placed in the incubator at 37.5°C to dry the sample. Besides, the researchers found that the residual of n-dodecane and n-hexadecane was extremely low when the samples were taken from the surface liquid; therefore, bioremediation was neglected in all of the surface liquid samples. A blank bioreactor simultaneously operated with the main bioreactor. The bioremediation of hydrocarbons was obtained by subtracting the total bioremediation of hydrocarbons in the main bioreactor from the remediation of hydrocarbons in the blank bioreactor.
In addition, variations in operating parameters, such as dissolved oxygen, temperature, pH, and the number of active bacteria, were determined. For this purpose, samples were collected at zero, one, two, and three days. Operating parameters were measured using digital DO meter (Model parker, 1987), pH meter (Model 51- Japan), and thermometer (20, 21).
4. Results
4.1. Variations of pH
Table 2 shows the variations of pH during three days of bioreactor operating cycle. The pH ranges for consortium types A and B were 5.3 to 7.5 and 6.5 to 7.6, respectively. After three days, the pH values for consortium types A and B decreased to 2.2 and 1.1, respectively.
4.2. Dissolved Oxygen
Table 2 provides the variations of dissolved oxygen during three days of bioreactor operating cycle. Based on the table, the dissolved oxygen ranges for consortium types A and B were 3 to 7 mg/L and 5 to 7.1 mg/L, respectively. After three days, the dissolved oxygen in bioreactors A and B decreased to 4 and 2.1 mg/L, respectively.
4.3. Temperature
Table 2 shows the variations of temperature during three days of bioreactor operating cycle. As shown in Table 2, the temperature ranges for bioreactors A and B were 25.5 to 29°C and 25.5 to 27°C, respectively. After three days, the temperature for bioreactors A and B increased to 3.5°C and 1.5°C, respectively.
4.4. Density of Active Bacteria
Table 2 shows the variations in density of active bacteria during three days of bioreactor operating cycle. Based on Table 2, the number of active bacteria ranges for bioreactors A and B, respectively, were 104 to 106 and 104 to 105 CFU/mL, respectively. After three days, the number of active bacteria for bioreactors A and B increased to 9 × 104 and 99 × 104 CFU/mL, respectively.
| Consortium Type | Time (Days) | |||
|---|---|---|---|---|
| Zero Day | First Day | Two Days | Three Days | |
| pH | ||||
| A | 7.5 | 6.5 | 6 | 5.3 |
| B | 7.6 | 7.2 | 6.7 | 6.5 |
| Dissolved oxygen (mg/L) | ||||
| A | 7 | 6.2 | 4.6 | 3 |
| B | 7.1 | 6.7 | 5.9 | 5 |
| Temperature (°C) | ||||
| A | 25.5 | 26.5 | 27.3 | 29 |
| B | 25.5 | 25.9 | 26.7 | 27 |
| Number of active bacteria (CFU/mL) | ||||
| A | 1×104 | 3×104 | 5×105 | 1×106 |
| B | 1×104 | 2×104 | 7×104 | 1×105 |
4.5. Bioremediation Study
Table 3 shows simultaneous bioremediation of n-hexadecane and n-dodecane by consortium types A and B. As depicted in Table 3, the minimum, average, and maximum simultaneous bioremediation of n-hexadecane by consortium type A was 6.64%, 12.79 %, and 17.61%, whereas this was 4.65%, 9.94%, and 13.22% by consortium type B. Besides, the minimum, average, and maximum simultaneous bioremediation of n-dodecane by consortium type A were 10.97, 18.77, and 28.55 percent, while they were 8.9, 14.71, and 19.24 percent by consortium type B.
| Type of Consortium | Simultaneous Bioremediation (%) | |||
|---|---|---|---|---|
| Zero Day | First Day | Two Days | Three Days | |
| N-hexadecane with consortium type A | 0 | 6.6 | 14.1 | 17.6 |
| N-hexadecane with consortium type B | 0 | 4.6 | 11.9 | 13.2 |
| N-dodecane with consortium type A | 0 | 10.9 | 16.7 | 28.5 |
| N-dodecane with consortium type B | 0 | 8.9 | 15.9 | 19.2 |
5. Discussion
Soil pH is important for enzyme activity (9, 27). The selection of pH depends on the organisms used for biodegradation (28). In the present study, after three days, pH values were decreased to 2.2 and 1.1 for consortium types A and B, respectively. The decrease in pH could attribute to the intermediate material produced by the consortium. In most studies, researchers reported that alkane degradation occurs at neutral pH. The pH of soil according to soil composition and history ranged from around 2.5 to around 10 (29). Schauer pointed out in his review that the oxidation rates of hydrocarbons varied only slightly at pH values between 5 and 8, whereas oxidation and even toxicity of organic acids, microbial hydrocarbon metabolism products, were clearly dependent on pH (30). In another study, Rhodococcus erythropolis strain NTU-1 was incubated in fed-batch bioreactor for bioremediation of diesel and petroleum. After six days of incubation with diesel, the pH decreased from 7 to 4.3, while in incubations with petroleum, the pH decreased from 7 to 5. In addition, pH changes were related to cell growth (31). It is known that pH changes of the medium affect the net charge of polysaccharides, phosphates, and amino groups on a cell’s surface (32). Also, results of the study of Dastgheib et al. showed that biodegradation of polycyclic aromatic hydrocarbons with Alcanivorax dieselolei strain QTET was extremely unstable at high pH. The range of its pH tolerance was limited (pH 6 to 8) and its optimum growth was at neutral pH (33).
Dissolved oxygen is essential for biodegradation (23). Because there is a high concentration of hydrogen and carbon, yet small amounts of oxygen in petroleum compounds (34), 3 to 4 mL of dissolved oxygen are required to oxidize 1 mL of hydrocarbons to CO2 and H2O (35). For aerobic bacteria, stoichiometrically, 3.1 mg/mL of oxygen is needed for the biodegradation of 1 mg/mL of hydrocarbons, regardless of the total mass of bacteria (36). In this study, after three days, the dissolved oxygen for consortium types A and B decreased to 4 and 2.1 mg/L, respectively. In agreement with other reports, such as that of Venkata Mohan et al. (5) and Juneson et al. (37), the activity of microorganisms and the rate of oxygen uptake increased.
Temperature has a noticeable effect on the capability of microorganisms for degradation of petroleum hydrocarbons. In addition, with rising temperature, solubility of oxygen is reduced, and consequently metabolic activity of aerobic microorganisms is also reduced. Most oil-degrading organisms are active in the meso-thermal ranges (20°C to 35°C) and have the best degradation rates at these temperatures (35). Some exceptions exist; for example, Rueter et al. (38) described a slightly thermophilic anaerobic strain (TD3) that degrades alkane under sulphate-reducing conditions; Klug and Markovetz (39) reported on thermophilic bacteria, which can degrade n-tetradecane. Also, industrial alkane degradation at high temperatures (e.g. 65°C to 70 °C) has been reported (40). Based on the current results, the temperature for consortium types A and B increased to 3.5°C and 1.5°C, respectively after three days. There was a direct relationship between temperature and activity of microorganisms, thus microorganisms activity increases with increasing temperature and conversely (41).
The findings of this study indicate that After three days, the number of active bacteria for consortium types A and B increased to 9 × 104 and 99 × 104 CFU/mL, respectively (Table 2). Genthner et al. reported that almost all PAHs are degraded at 15°C and at an oxygen level of 4 ppm, and at 40°C most PAHs are degraded at 0 ppm oxygen level (42). The increase in the number of active bacteria can be due to further adaptation of bacteria to the bioreactor and greater growth. In addition, it can concluded that bacteria type A were more compatible with oil hydrocarbons than type B. Genthner et al. reported that almost all PAHs are degraded at 15°C and at an oxygen level of 4 ppm, and at 40°C most PAHs are degraded at 0 ppm oxygen level (42). A few researchers showed that the number of active bacteria was 5.25 × 105, 1.76 × 106 and 5.11 × 105 cells per mL of soil. Besides, they showed that these species can degrade n-hexadecane up to a concentration of 120 ppm and a consortium of species could degrade n-hexadecane faster than they do separately (43).
Maximum simultaneous bioremediation of n-hexadecane by consortium type A and B was 17.61 and 13.22 percent. In addition, maximum simultaneous bioremediation of n-dodecane by consortium type A and B was 28.55 and 19.24 percent (Table 3). On the other hand, the bioremediation of n-hexadecane by consortium type A on days one, two, and three was 1.99, 2.17, and 4.39 percent greater than type B. The bioremediations of n-dodecane by consortium type A on days one, two, and three was 4.33, 2.67, and 10.94 percent greater than type B (Table 3). Based on the results, it can be concluded that consortium type A had better adaptation with bioreactor conditions than type A. In addition, bioremediation of n-dodecane during the three days of operation cycle was greater than n-hexadecane (Table 3). Considering the molecular formula of n-dodecane (C12H26), with lower carbon and hydrogen than n-hexadecane (C16H34), it can be suggested that the bacteria had a higher potential for bioremediation of n-dodecane than n-hexadecane. In Lopez et al.’s study, the findings showed that bioremediation was significantly influenced by different bioavailability (44). Also, Sun et al. (45) studied the simultaneous bioremediation of n-hexadecane and phenol. They found the strains were capable of simultaneous bioremediation of phenol and n-hexadecane in the mineral medium. However, the strains preferred phenol to n-hexadecane. Also, the coexistence of phenol and n-hexadecane was outperformed in the growth of strains in comparison with when they were used individually (45). A simulation test was conducted to study biodegradation of aromatic hydrocarbons (phenanthrene and anthracene) and aliphatic hydrocarbon (n-hexadecane) by native microorganisms, when the soil contained the test hydrocarbons individually or in coexistence. The results showed that coexistence of phenanthrene and n-hexadecane could serve as a co-metabolic substrate and promote biodegradation of phenanthrene, lessening the half-life of phenanthrene by 44% in comparison with when phenanthrene existed individually (46).
5.1. Conclusions
The results showed that the maximum simultaneous bioremediation of n-hexadecane and n-dodecane by consortium type A was 17.61 and 28.55 percent, respectively. In addition, the maximum simultaneous bioremediation of n-hexadecane and n-dodecane by consortium type B was 13.22 and 19.24 percent, respectively. Bioremediation of n-hexadecane and n-dodecane by bacterial consortium Type A (isolated from contaminated soil with oil) was significantly greater than type B (isolated from compost). In general, the bioremediation of n-dodecane during three days of the reactor’s running cycle was greater than n-hexadecane. The findings of this study showed the simultaneous bioremediation of n-hexadecane and n-dodecane in an S-SBR, using two types of bacterial consortium (type A and B) during a three-day period, was relatively satisfactory.