1. Background
Energy production from fossil fuels coincides with high CO2 emission, which is a gas responsible for climate change. In addition, population growth necessitates more energy production (1). The U.S. EIA (Energy Information Administration) reports that around 515-530 EJ (exajoules, 1018 J) energy was used worldwide in 2008 with an increasing trend (2). The hydrogen gas (H2) as the only carbon-free fuel has a high energy content (122 kJ/g; 2.75 times more than the energy content of hydrocarbon fuels) and produces only water when combusts (3, 4).
As the cleanest fuel gas, hydrogen is produced in processes such as natural gas, oils, and coal combustion and electrolysis (4), the processes mostly based on hydrocarbon fuel combustion; thus, the concern of air pollution remains more or less. Biohydrogen production from organic waste is one of the most promising alternatives. Dark fermentative hydrogen production is a complicated anaerobic process in the absence of sunlight that uses organic compounds as electron donors and acceptors (5, 6).
In waste treatment applications, biohydrogen production comes from mixed culture systems rather than pure cultures. Mixed cultures allow for bioprocessing of organic material in non-sterile environments and offer a greater ability to use mixed substrates due to higher microbial diversity. Mixed culture systems are easier and less expensive in operation, and facilitate continuous processing (7-9). However, the application of mixed cultures needs the enrichment of hydrogen-producing bacteria (HPB) attained through pretreatment methods. Indeed, in the anaerobic process, biohydrogen is produced by the inhibition of methanogenesis bacteria activity and enriching the HPB using sludge pretreatment. The overall efficiency and start-up period of biohydrogen-producing reactors can be affected by initial inoculum pretreatment (10-13).
In the literature, different methods have been suggested for mixed cultures pretreatment, including acid, alkaline, and heat shock, organic loading shock, and 2-bromoethanesulfonate acid (BESA) addition (10, 11, 14). Studies reported different methods as the best pretreatment for hydrogen production. For example, Wang and Wan introduced the heat shock method as the best pretreatment among other methods including acid shock, base shock, aeration, and chloroform addition for enriching HPB from digested sludge (15). Zhu and Béland examined the effects of heat, acids, bases, aeration, 2-bromoethanesulfonic acid (BESA), and iodopropane on the microbial seed preparation and concluded that specific methanogens inhibition was obtained by BESA and iodopropane pretreatment methods (5). O-Thong et al. studied BESA, base, load-shock, acid, and heat treatments for HPB preparation, demonstrating that the load-shock treatment method was the best for enriching HPB from mixed anaerobic cultures (16).
2. Objectives
There is no general agreement with the best pretreatment method for enriching HPB from seed sludge. Therefore, this study aimed to evaluate the effects of chemical pretreatment methods including different acids (H2SO4, H3PO4, HNO3, and HCl) and different bases (NaOH and KOH) on mixed cultures (anaerobic sludge) to enhance fermentative biohydrogen production in glucose medium.
3. Methods
3.1. Inoculum and Pretreatment Method
In this study, anaerobically digested sludge was used as a mixed culture source. Anaerobic sludge was obtained from an anaerobic sludge digester from the South Municipal Wastewater Treatment Plant (Tehran, Iran). In order to remove large particles and debris, the parent sludge was sieved with standard mesh No. 16 with 1.19 mm pore size (DG scientific production Co.). The raw sludge had a pH of 7.75 ± 0.1 with volatile and total suspended solids concentrations of 16.84 ± 3.4 and 32.56 ± 6.58 g/L, respectively (MLNSS/MLSS ratio: 0.52). Prior to use, the anaerobic sludge was subjected to acid or alkaline pretreatment to enrich the HPB. In the acid pretreatment method, four strong acids including HCl (35%), HNO3 (65%), H2SO4 (95%), and H3PO4 (85%) were used (6) to reduce the pH of the anaerobic sludge to 3.0. After 24 h, the sludge was adjusted to pH 7.0 with NaOH and KOH addition (6 M). In the alkaline pretreatment method, the pH of the raw sludge was adjusted to 12 with NaOH and KOH (12 M) and maintained for 24 h; then, it backed to pH 7.0 with the addition of concentrated H2SO4.
3.2. Biohydrogen Production Test (BHPT)
Stirred glass flasks were used for batch experiments with a total volume of 500 mL comprising a working volume of 400 mL and headspace of 100 mL for gas accumulation. Each flask contained 200 mL of acid or alkaline-pretreated anaerobic sludge and 200 mL of substrate medium. Glucose was used as a sole substrate at 4 g/L. We also added nutrients (NH4Cl, 76.45 mg/gCOD and KH2PO4, 10.00 mg/gCOD) and trace elements as mg/gCOD (K2HPO4, 25.32; FeCl3, 1.021; CaCl2.2H2O, 2.06; MgSO4.7H2O, 2.14; MnCl2.2H2O, 0.34; CoCl2.6H2O, 0.092; NiSO4.6H2O, 0.0763; ZnSO4, 0.0592; Na2MoO4.2H2O, 0.0822; CuCl2.2H2O, 0.016; and H3BO3, 0.020). In addition, yeast extract and peptone were used at 36 mg/L (17). For each pretreatment, batch experiments were conducted in triplicate at 37 ± 0.2°C using a hot water bath stirred at 150 rpm (360 s idle and 30 s mixing) via magnetic stirrers. The liquid displacement technique was used for measuring the biogas production volume. All batch tests were conducted without external buffer addition.
3.3. Analysis
Alkalinity, solution pH, and soluble chemical oxygen demand (sCOD) were analyzed according to the standard methods for the examination of water and wastewater (18). The hydrogen percentage of the produced biogas was quantified by a hydrogen analyzer (COSMOS-XP-3140 model, Japan) (12).
4. Results
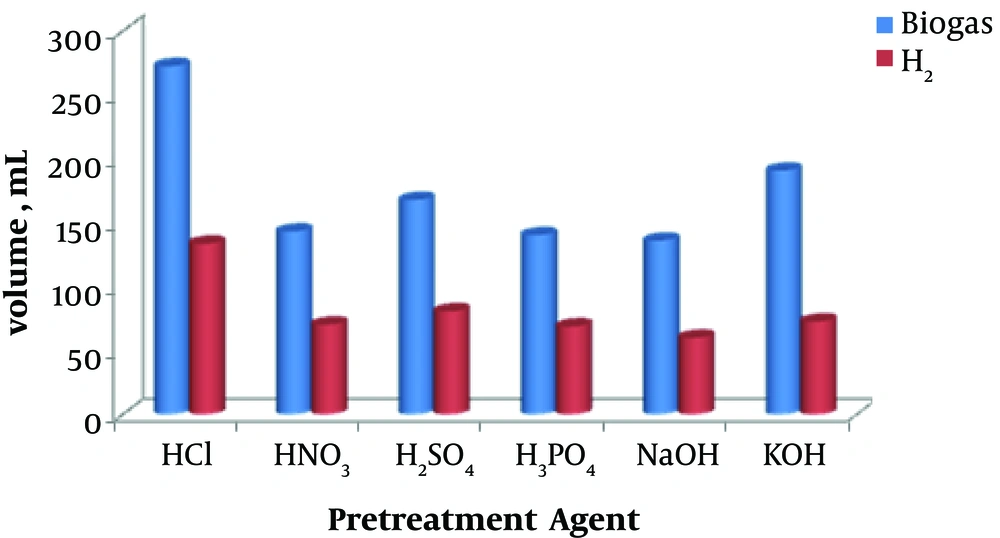
The volumes of produced biogas and hydrogen gas are depicted in Figure 1. As can be seen, the highest cumulative biohydrogen production (133.16 mL) was obtained with acid pretreatment method using HCl while the lowest biohydrogen production (59.84 mL) was observed with NaOH pretreatment. The volumetric hydrogen percentage in the biogas was 49% for HCl, HNO3, and H3PO4, 48% for H2SO4, 44% for NaOH, and 38% for KOH. Hence, the order of six methods for the cumulative biohydrogen production is as follows: HCl > H2SO4 > KOH > HNO3 > H3PO4 > NaOH.
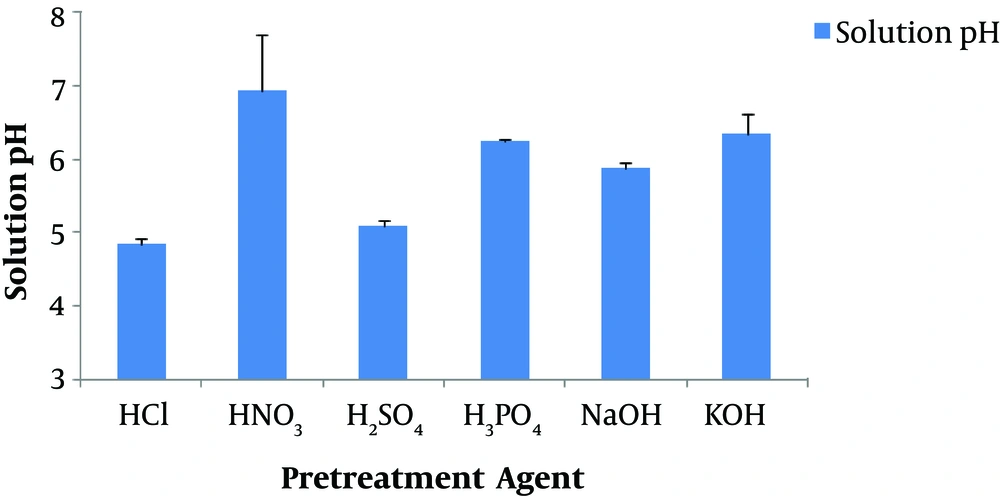
Figure 2 shows the variation of solution pH at the end of incubation. In all the BHPTs, the pH was maintained within the range of 4.9 and 6.9. The solution pH was the lowest in HCl and H2SO4 pretreatment methods (almost less than 5). This drop in pH could be attributed to the high accumulation of volatile fatty acids (VFAs). The order of methods for solution pH is as follows:
HCl (4.9) > H2SO4 (5.1) > NaOH (5.9) > H3PO4 (4.2) > KOH (6.4) > HNO3 (6.9)
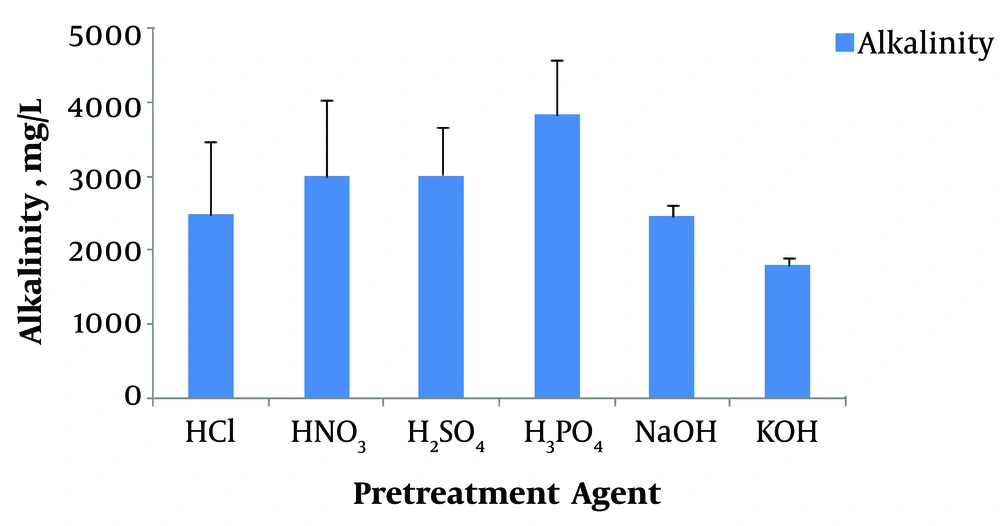
The results obtained for effluent alkalinity are shown in Figure 3. The effluent alkalinity was in the range of 1,800 to 3,800 mg/L as CaCO3. However, the initial buffering capacity was in the range of 4200 - 4500 mg/L. The drop in the alkalinity can be attributed to VFAs produced during the biohydrogen production process. As seen in Figures 2 and 3, decreasing alkalinity coincided with decreasing solution pH. The overall order of sludge pretreatment methods for alkalinity decrease is as follows: KOH > NaOH > HCl > HNO3 > H2SO4 > H3PO4.
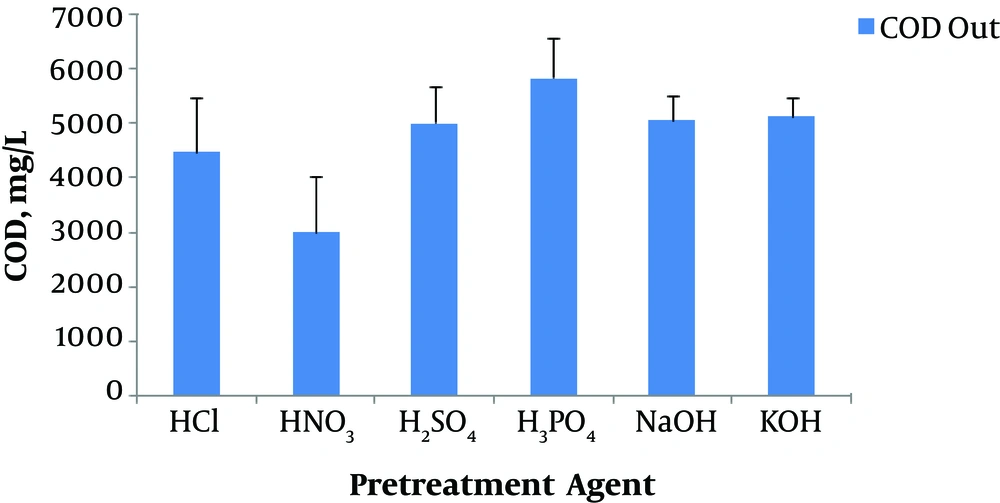
Substrate residuals (as effluent sCOD) at the end of incubation time are depicted in Figure 4. As seen, the effluent sCOD was in the range of 3,000 to 4,800 mg/L. In all the BHPTs, the effluent sCOD was higher than the influent sCOD except for HNO3 pretreatment. The order of methods for effluent sCOD is as follows: H3PO4 > KOH > NaOH > H2SO4 > HCl > HNO3. This observation was presumably related to carbohydrate sugars extracted from the parent sludge during the pretreatment processes.
5. Discussion
Mixed cultures (sludge, soil, manure, etc.) usually contain a wide variety of microorganisms including HPB and consuming microorganisms. In order to enhance the biohydrogen production potential, microbial communities are subjected to pretreatment methods. The activity of methanogens bacteria in mixed cultures can be suppressed by heat treatment or extreme alkaline or acidic pH (11, 19). In addition, a chemical inhibitor (BESA) specific to methanogens (5) has been used to enhance the biohydrogen production (10). As stated before, acid or base addition has a lower efficiency for HPB enrichment than heat pretreatment (11). However, the highest yield of biohydrogen was achieved with acid pretreatment (19, 20). In addition, successful methanogens inhibition was not reported via alkaline pretreatment (10, 11, 20).
The pretreatment methods are not always advantageous. The enrichment of acid-treated parent sludge culture resulted in higher biohydrogen production than alkaline-treated mixed cultures. These results are in line with conclusions made in previous studies (14, 19, 21).
The activity of microorganism enzymes is highly pH-dependent and the maximum activity of each enzyme is attained in a specific range of solution pH (22). Solution pH presents as a critical factor affecting biohydrogen production. The fermentation pathways of biohydrogen process are pH sensitive and are subject to final products including soluble and gaseous end-products (23). It has been reported that suboptimal pH leads the biohydrogen process to shift from the acidogenic to solventogenic pathway (24) or extends the lag phase before exponential biohydrogen production (25). The prompt variation in operation parameters including solution pH, hydraulic retention time, and temperature leads to a significant amount of lactate production, demonstrating the non-adaptation nature of mixed cultures to new conditions (24, 26). In our study, the optimal pH was within 5.0 to 5.5, which is in line with previous reports (27-29).
The alkalinity of anaerobic environments has been accepted as an indicator of VAF production that is associated with the buffering capacity of the system (30). According to Benefield and Randall (1980), the concentration of VFAs in the range of 900 to 2800 mg/L as acetic acid leads to a drop in solution pH. Based on the literature, the low alkalinity can cause (i) dropped solution pH, (ii) low biohydrogen content of biogas, (iii) low biohydrogen production rate, and (iv) prolonged lag phase of biohydrogen production (31, 32). Luo et al. reported that the hydrogen consumption and its rate increase with the drop in pH (33), demonstrating the need for adequate alkalinity to inhibiting homoacetogenesis bacteria at low pH (34).
5.1. Conclusions
In this study, we examined the effect of acid and alkaline sludge pretreatment on the enrichment of HPB for biohydrogen production in batch experiments. The results showed that the highest cumulative biohydrogen production (133.16 mL) was obtained with acid pretreatment using HCl while the lowest biohydrogen production (59.84 mL) was observed under NaOH pretreatment. The alkalinity drop coincided with decreasing solution pH. The overall alkalinity drop order of the sludge pretreatment methods was as follows: KOH > NaOH > HCl > HNO3 > H2SO4 > H3PO4.