1. Background
Carcinogenic compounds are a major cause of death worldwide, so 7,600,000 deaths occurred in 2008, and 13.1 million deaths are predicted by 2030, of which cancer is the main cause (1). Anticancer drugs are used in controlled amounts and conditions in medical centers. Platinum complex drugs, especially cisplatin, are used as platinum-containing chemotherapy drugs to decrease or stop the growth of cancer cells in various types of cancer (2). In general, it is desirable that such drugs act specifically by damaging the genetic resources of tumor cells, inhibiting their synthesis, and accelerating the induction of apoptotic cell death (3). In addition, the drugs have effective molecular mechanisms, including the induction of oxidative stress (OS), which increases the generation of reactive oxygen species (ROS) in tumor cells (4). Therefore, there is a possibility of lipid peroxidation and reduction of cellular antioxidant defense processes such as glutathione in healthy cells, which can have significant side effects such as nausea, diarrhea, vomiting, and nephrotoxic, neurotoxic, hematotoxic, hepatic, and cardiac effects (5-7).
The main environmental source of platinum complex drugs is the feces and urine of patients undergoing chemotherapy, which are excreted into municipal wastewater systems. Some findings suggest the presence of drug residues in aquatic environments. However, the specific effect of drug residues on the general population is unclear (8-10). Therefore, in recent years, measures have been taken to evaluate the risks of anticancer drugs in the environment (10). According to studies conducted in Iran (in medical centers in Qom and Urmia provinces), typically, 15 - 25% of the anticancer drugs used by patients admitted to the aforementioned centers were platinum-containing compounds. Thus, the high consumption of anticancer drugs in Iran and the plans of the Ministry of Health to develop oncology wards in medical centers must be taken into account, as must be the issue of the release of drug residues into the environment due to the lack of a proper management system for collection, elimination, and disposal of residual drugs. Moreover, the lack of appropriate wastewater treatment systems to remove drug contaminants, the health consequences, the need to assess the rate of entry, environmental monitoring of platinum-containing compounds in municipal wastewater networks, prediction of drug concentrations in water sources, and studying the risk and environmental toxicity of drugs are of great importance (11).
2. Objectives
Considering the health and environmental toxicity aspects of the release of platinum anticancer drugs in the environment, this study aimed to monitor and evaluate the environmental risks of these compounds in the municipal wastewater effluent of Qom City.
3. Methods
3.1. Study Setting
Environmental monitoring and ecological risk assessment of platinum complex drugs in this study were designed and carried out in two stages. In the first stage, the concentrations of platinum complex drugs (oxaliplatin, carboplatin, and cisplatin) in the Qom City wastewater treatment plant effluent were determined. In the next stage, an ecological risk assessment was done. Part of the wastewater treatment system of Qom city, which utilizes biological treatment processes, is designed to receive urban and hospital wastewaters with a population of 250,000 people and a flow rate of Q = 0.7 m3/s.
3.1.1. Sampling Process, Preparation, and Analytical Method
This study collected and analyzed 33 effluent samples from the municipal wastewater treatment plant based on 24-h composite sampling. The sampling sites of this research were in the Qomrud region of the studied province. Sampling sites were selected from the open canals carrying treated wastewater and raw urban wastewater along a length of 15 km in the vicinity of the Qomrud region (Figure 1).
Mass Chromatography-Mass Spectrometry (LC-MS/MS) determined the concentrations of platinum compounds in the samples. The calibration curves were drawn with 1, 5, 10, 25, and 50 ng/L concentrations of carboplatin, oxaliplatin, and cisplatin. The correlation coefficients > 0.9998, 0.9987, and 0.999 were obtained for oxaliplatin, carboplatin, and cisplatin, respectively. In all cases, the Relative Standard Deviation in percentage (RSD%) of platinum cytotoxic compounds (n = 5) was below 15%. The extraction recovery was determined by analyzing five concentrations (1.5, 10.25, and 50.00 ng/mL). In this study, required sensitivity by recovery of more than 50% was considered. The limit of quantification (LOQ) and limit of detection (LOD) were calculated using the following equation:
LOQ = 10σ/S, LOD = 3:3σ/S,
Where σ is the standard error of the intercept, and S is the slope of the standard addition calibration curve. Multiple Reactions Monitoring (MRM) was used for the detection. For the platinum complex, [M+H]+ ions were monitored at m/z as the product ion, and the result is shown in Table 1 (10).
| Compound | Retention Time, min | Segment | ESI | Product Ion | MS/MS Transition |
|---|---|---|---|---|---|
| Cisplatin | 5.3 | 2 | ESI+ | [M+H]+ | 300 > 248 |
| Carboplatin | 5.3 | 1 | ESI+ | [M+H]+ | 372 > 355.0 |
| Oxaliplatin | 5.3 | 1 | ESI+ | [M+H]+ | 398 > 96.0 |
Specific Multiple Reactions Monitoring Conditions for Determination of Platinum Cytotoxic Drugs by Mass Chromatography-Mass Spectrometry
3.1.2. Ecological Risk Assessment of Platinum Complex Drugs
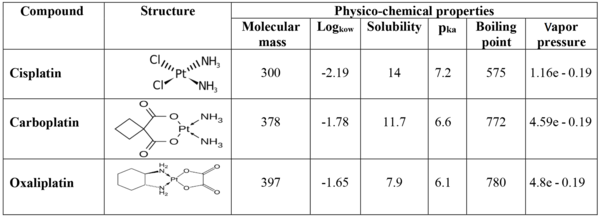
In order to conduct an ecological risk assessment of cytotoxic drugs, the physicochemical properties contributing to the fate and transport of chemicals in the environment were considered by a theoretical model (EPI Suite 4.1). The characteristics and the environmental fate of Pt complex drugs are shown in Figure 2. The solubility factor, log (Kow), and vapor pressure showed that the risk of platinum compounds in aquatic environments is critical (11).
Physicochemical parameters, such as the dissociation constant (pKa), the octanol-water dissociation coefficient (Kow), solubility, Henry's coefficient, and vapor pressure, can be used for the drug risk analysis. The range of pKa values determines which compound dissociates at a given pH. An octanol-water dissociation coefficient of less than one (log Kow < 1) indicates that the chemical compound has high mobility in the aqueous medium and, therefore, exhibits the possible behavior of an anticancer compound with this characteristic, remains in the aqueous phase and is not absorbed by sediments or sludge in the environment. The risk assessment of a drug complex with log Kow > 4.5 should only be considered regarding degradability, biodegradability, and toxicity. In general, cytotoxic and anticancer drugs are mainly highly soluble in water with low log Kow values, which is important in terms of increasing access and exposure to drug residues in drinking water sources because drugs have a low vapor pressure under normal conditions (25ºC), and accordingly, cannot volatilize in the environment (12-14).
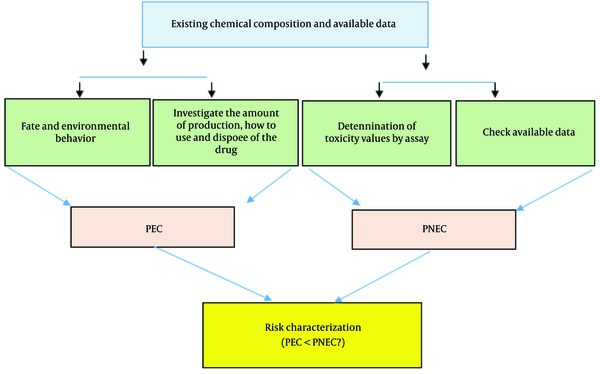
Ecological risk assessment of Pt compounds was done using the following diagram (Figure 3).
In the predicted environmental concentration (PEC) determination model (which predicts the concentration of drug compounds in the environment), according to quantities of platinum complex drugs in the treated wastewater effluent, the highest measured values of each platinum compound, including cisplatin, carboplatin, and oxaliplatin, with the worst case scenario were considered as PEC values. The reliability of the PEC value is based on the ratio of PEC/MEC estimated with an acceptable consistency for the ratio 0.2 - 4 (3).
Risk estimate in the PEC model was used by risk quotients (RQ) or hazard quotients (HQ). This ratio is determined by calculating the PEC and determining the concentration at which we do not expect unacceptable adverse effects (predicted no-effect concentration, PNEC). The PNEC values are estimated through the toxicity threshold concentration ratio (LC50 or EC50) and the safety factor (f = 100). In this study, the PNEC of platinum complex drugs for aquatic animals was taken from literature data as 1.22 µg/L (3).
The present study calculated RQ for wastewater treatment plant effluent in surface water (RQsw).
RQ < 1.0 indicates very low and insignificant risk;
1.0 ≤ RQ < 10 indicates a low potential for adverse effects;
10 ≤ RQ < 100 shows the ability to create high adverse effects (15, 16).
Equations 1 and 2 demonstrate how to determine the risk ratio.
Equations 1: RQ=PEC/PNEC
Equations 2: RQ=PEC/EC50/f
4. Results
Statistical analysis results are presented in Tables 2 and 3 and Figures 4 and 5.
| Compound | Linearity (R2) | LOD (ng/L) | LOQ (ng/L) | Recovery% | RSD% (n = 5) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 ng/mL | 5 ng/mL | 10 ng/mL | 10 ng/mL | 50 ng/mL | |||||
| Cisplatin | 0.999 | 0.1 | 0.3 | 0.70 | 6.5 | 8.2 | 5.8 | 5.6 | 9.1 |
| Carboplatin | 0.999 | 0.013 | 0.4 | 0.78 | 6 | 6.4 | 7.8 | 6.9 | 8.9 |
| Oxaliplatin | 0.999 | 0.09 | 0.027 | 0.74 | 7.5 | 7.7 | 9.9 | 8.8 | 7.9 |
Method Validation and Quality Control Parameters of Platinum Complex Drugs
| Drug Compound | MEC (µg/L) | PEC (µg/L) | PEC/MEC | PNEC(µg/L) | RQSw |
|---|---|---|---|---|---|
| Cisplatin | 0.19 | 0.4 | 2.1 | 122 | 32 E-4 |
| Carboplatin | 0.22 | 0.36 | 1.63 | 122 | 29 E-4 |
| Oxaliplatin | 0.12 | 0.26 | 2.16 | 122 | 21 E-4 |
Ecological Risk of Exposure to Platinum Complex Drugs
5. Discussion
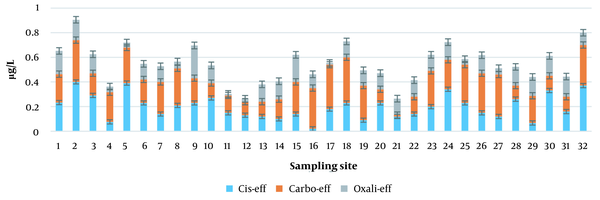
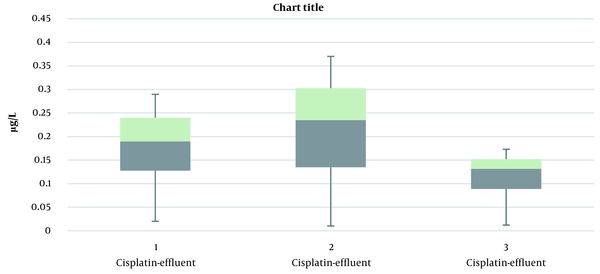
As described in the materials and methods section and based on the results presented in Table 2, the instrumental LOQ and LOD for cisplatin, carboplatin, and oxaliplatin were determined. According to these results, the LODs of cisplatin, carboplatin, and oxaliplatin were calculated to be 0.1, 0.013, and 0.09 µg/L, respectively. Similarly, the LOQs of cisplatin, oxaliplatin, and carboplatin were 0.3, 0.027, and 0.4 µg/L, respectively. Figure 4 indicates the concentration of platinum complex drugs in the municipal wastewater treatment plant effluent. According to this figure, the concentrations of oxaliplatin, carboplatin, and cisplatin in the effluent of the wastewater treatment plant were 0.12 ± 0.059, 0.22 ± 0.094, and 0.19 ± 0.098 µg/L, respectively. The results show that the pattern of changes in the concentration of platinum pharmaceutical compounds in the municipal wastewater treatment plant effluent is the same at the sampling points. Figure 5 shows the box plot of cisplatin, carboplatin, and oxaliplatin in the effluent samples, in which the labeled bars indicate significant differences among samples (P < 0.05).
Ecological risk of exposure to platinum complex drugs and RQsw calculated for cisplatin, carboplatin, and oxaliplatin (32E-4, 29E-4, and 21E-4, respectively) show that these platinum complex drugs could have no significant risk on aquatic organisms. In this study, ΣRQsw was estimated at < 1 (Table 3). In a study of the ecological risk assessment of 98 pharmaceuticals, including platinum drugs, in Indian surface water and wastewater, anticancer drugs were detected in domestic wastewater effluent at ng/L (17). The difference in the concentrations of platinum compounds between Indian wastewater and the wastewater in Qom City in the present study is due to the use of higher doses of platinum complex drugs in Qom City. By examining the characteristics and environmental behavior of platinum pharmaceutical compounds according to Figure 2, it is inferred that these compounds have low biodegradability (18, 19). In the study by Tripathi et al. on the environmental remediation of antineoplastic drugs, the degradation or deactivation of antineoplastic drugs in the wastewater and environment was highly influenced by the individual physicochemical behavior of antineoplastic drugs and their transformation products, which confirms the findings of the present study (20). By comparing the study findings of Ghafuri et al. on the toxicity characterization and environmental risk assessment of platinum cytotoxic drugs in hospital effluents with the results of the present study, it can be concluded that high concentrations of platinum complex drug residues were removed in the municipal wastewater system and treatment plant by adsorption to sludge and other particles (14). In a study on removing platinum compounds in the biological wastewater treatment process, Lenz et al. showed that cisplatin and carboplatin could be removed by 51% and 63% via adsorption, respectively (21). In a study on the removal of platinum complex drugs in a pilot-scale membrane bioreactor (MBR) system, the removal of these drugs due to adsorption onto sewage sludge was determined to be 60% on average (22). Based on the findings presented in Table 3 concerning the ecological risk of exposure to platinum complex drugs, the obtained RQ shows that the risk of environmental exposure is negligible for all three Pt compounds.
In a study by Rowney et al. on the prediction of cytostatic drugs in wastewater effluents, the concentration of the carboplatin drug compound, with a probability of 90%, was determined to be less than 1 ng/L (23). The study by Franquet-Griell et al. on predicting the concentrations of cytostatic drugs in sewage effluents and surface waters of Catalonia reported that the concentration of compounds of common drugs used in the effluent from the municipal wastewater treatment system was in the range of 2.7 - 0.06 µg/L (24). It seems that the differences in the results of the above study and those of the present study in terms of the determined concentrations are related to the physicochemical properties of drug compounds and their behavior in the aqueous medium, their dose and excretion rate, and the sampling process (23, 24). In the study by Daouk et al. on drug compounds in the wastewater of a university hospital in Switzerland and the prediction of platinum-based anticancer drugs, PEC values were calculated, confirming the results of the present study (25). Cunningham et al. investigated the environmental exposure to drug compounds, including anticancer drugs, and calculated the tolerable daily intake values to estimate the amount without observable health effects through drinking water and fish consumption. These values showed low exposure risk compared with ambient PEC concentrations and were similar to the present study's finding (26). In a study by Besse et al., which predicted anticancer levels in surface waters using the PEC model and assessed exposure risk, the PEC value for cisplatin, oxaliplatin, and carboplatin was 0.52 ng/L (27).
The study focused on the platinum complex compounds in hospital wastewater that cause ecotoxicity of wastewater. This study showed that high-performance liquid chromatography-mass spectrometry (HPLC-MS)/MS could be an important tool in identifying these compounds.
In order to reduce the environmental toxicity of hospital wastewater, appropriate methods of cytotoxic waste management and raising awareness among the related staff should be considered. According to the additional findings from the study of environmental effects and health consequences of platinum complex drugs, these compounds can cause toxicity in aquatic environments, including hospital effluents, sewage, and groundwater.
5.1. Conclusions
Cytotoxic platinum drugs are life-saving for cancer patients. Unfortunately, recent studies have demonstrated that these drugs are not completely removed from the wastewater system. Complementary studies should be implemented, including the assessment of genotoxicity and toxicity effects of cytotoxic platinum drugs and the removal methods of residual platinum compounds. This study indicated that managing cytotoxic wastes from hospital oncology wards is vital for environmental pollution control. Considering the low efficiency of conventional wastewater treatment systems in removing platinum cytotoxic pharmaceutical compounds, using other methods to remove these compounds, such as advanced oxidation processes and membrane systems, is inevitable.