1. Background
Myocardial infarction (MI) is a significant global health issue, causing illness, mortality, and stress. It is estimated that over 3 million individuals experience an ST-elevation myocardial infarction annually; however, non-ST elevation myocardial infarction affects more than 4 million individuals. Although MI was previously considered a disease that mainly occurred in developed countries, its prevalence is increasing in developing nations (1). The MI occurs when there is a reduction in myocardial perfusion sufficient to cause cell necrosis, often accompanied by thrombus formation in the coronary arteries. The causative event is the rupture of an atherosclerotic plaque, which exposes the blood to thrombogenic lipids and triggers the initiation of platelets and clotting factors in the blood. The most common coronary plaque leading to stenosis is a lipid-enriched core with a thin fibrous cap (2).
The MI refers to a heart attack caused by plaque build-up on the inner walls of arteries, decreased blood flow to the heart, and damage to the heart muscle due to hypoxia (3, 4). The etiology of MI is not limited to atherosclerosis, as several different causes can lead to this condition (5). Patients who have experienced an MI often present with chest pain, which can vary in severity and emotional impact due to the subjective nature of pain (6, 7). Thrombus formation at the base of a sclerotic plaque with an additional lumen often results in myocardial perfusion impairment during MI (8). Atherosclerosis can progress through a cyclic process of injury and repair, leading to increased plaque rupture and thrombosis (9).
Basically, oxidative stress is caused by reactive oxygen species (ROS) and is responsible for tissue destruction and different pathological conditions. The ROS family includes free radicals, such as superoxide and nitrogen dioxide, and non-radicals, such as hypochlorous acid, lipid hydroperoxide, and ozone (10). The ROS are important in mediating corporeal responses. Following exposure to environmental stressors, such as hypoxia, ROS production can disrupt endogenous antioxidant systems and cause myocardial tissue damage (11). Oxidative stress is the major cause of myocardium tissue damage. Free radicals have been linked to numerous diseases, such as heart diseases, certain cancers, aging, inflammation, and anemia. However, antioxidants fight against numerous diseases and disorders by neutralizing disease-causing free radicals. Antioxidant compounds have the ability to neutralize ROS, serve as a defense against free radicals, or prevent the production of ROS or oxygen radicals (11).
Endogenous antioxidants are classified into enzymatic or non-enzymatic antioxidants. Superoxide dismutase (SOD) is an enzymatic antioxidant and an important mechanism of defense in living organisms. Enzymatic antioxidants break and remove free radicals, for example, SOD and glucose oxidase (12). Non-enzymatic antioxidants are albumin, transferrin, and ferritin, which are defensive antioxidants in the plasma of human blood and play a vital role in the first line of body defense (13).
2. Objectives
The present study aimed to determine which biochemical parameters (i.e., lipid profile and cardiac profile) affect antioxidant assay at its highest level in MI patients.
3. Methods
An observational cohort study was conducted at the University of Lahore, Pakistan, and the 53 samples were collected from the Punjab Institute of Cardiology in Lahore and Rehmatul lil Alameen Institute of Cardiology in Lahore through the non-random sampling technique. To avoid the effect of confounding variables, the inclusion criteria were patients who were non-smokers and had a body mass index below 27 kg/m2.
3.1. Estimation of Biochemical Parameters
After taking the clinical history from patients, 5 ml of the blood sample was collected from the patients who were admitted to the intensive care unit (ICU) 24 hours ago, and the serum of the collected samples was separated on the high gravitational force of the centrifuge. All the procedures were performed under the World Health Organization’s standard operating procedures. Then, these serum samples of the suspected MI patients were examined on the cobas c 311 and cobas e 411 (Roche, Switzerland) chemistry analyzers for the evaluation of lipid profile (i.e., total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL), and triglycerides (TG)) and cardiac profile (i.e., troponin T, troponin I, creatine kinase MB (CK-MB), and creatine phosphokinase (CPK)) by Roche kits (Roche, Switzerland).
3.2. 2,2-Diphenyl-1-picrylhydrazyl Assay
After the evaluation of biochemical parameters, antioxidant activity was done to inhibit the oxidative stress in the confirmed patients’ samples using 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich, USA), sodium phosphate buffer (Honeywell Fluka, USA), and methanol solution (Sigma-Aldrich, USA). The DPPH reduction assay was performed by adding 400 μL of 0.1 mM working solution of DPPH and then adding 400 μL of 10 mM sodium phosphate buffer. Then, 20 μL of serum was added and incubated for 30 minutes at an ambient temperature of 21°C. The absorbance of the samples was measured at 520 nm by using UV-180 and U-2900 HITACHI spectrophotometers (Shimadzu, Japan) (14). The percentage of the DPPH scavenging effect was calculated by the following formula (15):
A0 = The absorbance of control
A1 = The absorbance of sample
3.3. Statistical Analysis
The analysis of the data was carried out using Statistical Package for the Social Sciences software (SPSS, version 25.0). Pearson correlation, mean, and standard deviation (16) were calculated to assess the results. A P-value < 0.05 was considered statistically significant.
4. Results
The 53 MI patients in this study were within the age range of 28-85 years. Moreover, 20 (37.7%) and 33 (62.3%) patients were female and male, respectively. The lipid profile was significantly elevated (TC: 380 mg/dL, TG: 320 mg/dL, VLDL: 46 mg/dL, LDL: 185 mg/dL) except for HDL, indicating a low level (45 mg/dL) in the MI patients (Table 1). Similarly, the cardiac profile (CPK: 29.55 u/L, CK-MB: 182 u/L, troponin T: 14560 pg/mL, troponin I: 26224.4 pg/mL) was also remarkably elevated in the MI patients (Table 2). The results were confirmed by the normal international values of the concerned biochemical parameters.
| Parameters | Patients (n = 53) | |
|---|---|---|
| Mean ± Standard Deviation | P-Value a | |
| Total cholesterol (mg/dL) | 228.68 ± 62.608 | 0.01 |
| Triglycerides (mg/dL) | 187 ± 52.77 | 0.01 |
| High-density lipoprotein (mg/dL) | 33.67 ± 6.30 | 0.001 |
| Low-density lipoprotein (mg/dL) | 119.05 ± 15.53 | 0.01 |
| Very-low-density lipoprotein (mg/dL) | 34.77 ± 5.10 | 0.01 |
a A P-value < 0.05 was considered significant.
| Parameters | Patients (n = 53) | |
|---|---|---|
| Mean ± Standard Deviation | P-Value a | |
| Creatine phosphokinase (u/L) | 487.00 ± 588.70 | 0.01 |
| Creatine kinase MB (u/L) | 42.37 ± 34.67 | 0.001 |
| Troponin T (pg/mL) | 2006.19 ± 3413.68 | 0.01 |
| Troponin I (pg/mL) | 3011.63 ± 5682.29 | 0.01 |
a A P-value < 0.05 was considered significant.
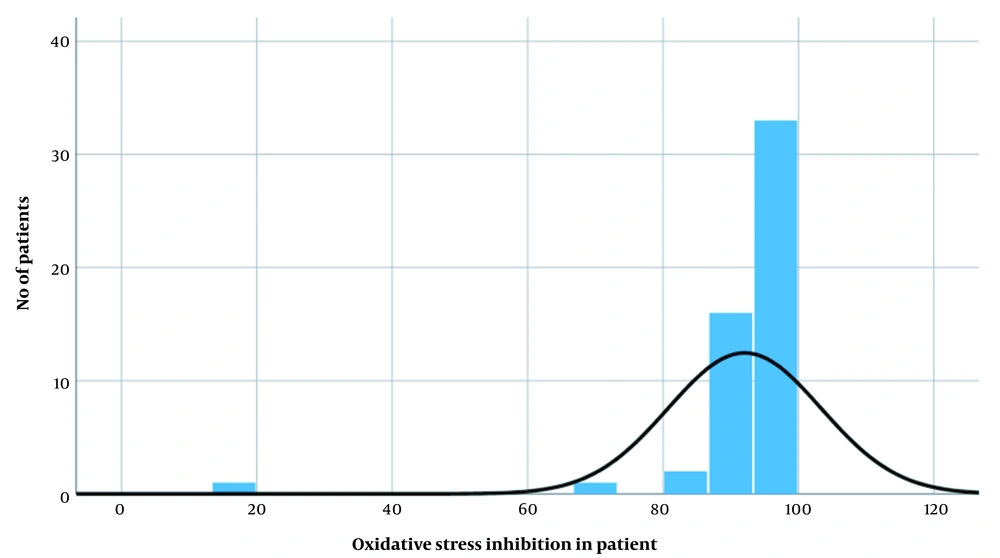
After the confirmation of MI, a DPPH assay was performed for the inhibition of oxidative stress, and as per the result, the maximum and minimum inhibition of oxidative stress were 100% and 18.56%, respectively, with a mean score equal to 91.73 ± 11.23. Therefore, the results of maximum inhibition revealed that the DPPH was a suitable agent against oxidative stress, as shown in Figure 1.
Table 3 is a correlation matrix table that shows the Pearson correlation coefficients between various biological markers, such as age, troponin I, troponin T, CK-MB, CPK, TC, TG, HDL, LDL, VLDL, and DPPH assay, a measure of antioxidant activity. The correlation coefficients range from -1 to 1, indicating the strength and direction of the linear relationship between the markers. A positive correlation coefficient indicates a direct relationship; nevertheless, a negative coefficient indicates an inverse relationship. The asterisks indicate the statistical significance of the correlations, with b and a indicating very strong and moderate correlations, respectively.
| Pearson Correlation | Age | Troponin I | Troponin T | Creatine Kinase MB | Creatine Phosphokinase | Total Cholesterol | Triglycerides | High-density Lipoprotein | Low-density Lipoprotein | Very-low-Density Lipoprotein | DPPH Assay |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.294 a | 0.250 | -0.038 | 0.013 | 0.342 a | 0.259 | -0.284 a | -0.191 | -0.233 | 0.067 | |
| Troponin I | 0.294 a | 0.702 b | 0.466 b | 0.392 b | 0.391 a | 0.283 a | -0.352 b | -0.182 | -0.421 b | -0.18 | |
| Troponin T | 0.250 | 0.702 b | 0.522 b | 0.383 b | 0.271 | 0.233 | -0.287 a | -0.322 a | -0.293 a | 0.048 | |
| Creatine kinase MB | -0.038 | 0.466 b | 0.522 b | 0.480 b | 0.250 | 0.278 a | -0.119 | -0.110 | -0.180 | -0.319 a | |
| Creatine phosphokinase | 0.013 | 0.392 b | 0.383 b | 0.480 b | 0.195 | 0.210 | -0.212 | -0.023 | -0.166 | -0.332 a | |
| Total cholesterol | 0.342 a | 0.319 a | 0.271 | 0.250 | 0.195 | 0.87 b | -0.607 b | -0.534 b | -0.583 b | 0.118 | |
| Triglycerides | 0.259 | 0.283 a | 0.233 | 0.278 a | 0.210 | 0.870 b | -0.610 b | -0.525 b | -0.593 b | 0.164 | |
| High-density lipoprotein | -0.284 a | -0.352 b | -0.287 a | -0.119 | -0.212 | -0.607 b | -0.61 b | 0.556 b | 0.564 b | -0.249 | |
| Low-density lipoprotein | -0.191 | -0.182 | -0.322 a | -0.110 | -0.023 | -0.534 a | -0.52 b | 0.556 b | 0.47 b | -0.185 | |
| Very-low-density lipoprotein | -0.233 | -0.421 b | -0.293 a | -0.180 | -0.166 | -0.583 b | -0.593 b | 0.564 b | 0.476 b | -0.142 | |
| DPPH assay | 0.067 | 0.018 | 0.048 | -0.31 a | -0.332 a | 0.118 | 0.164 | -0.249 | -0.185 | -0.142 |
Abbreviation: DPPH, 2,2-Diphenyl-1-picrylhydrazyl.
a Pearson correlation is significant at the 0.05 level (two-tailed).
b Pearson correlation is significant at the 0.01 level.
5. Discussion
The MI is the leading cause of illness and death worldwide (17). Although it mainly occurs in developed countries, it is becoming increasingly common in developing nations (18). The MI occurs due to reduced myocardial perfusion that causes cell necrosis, usually associated with thrombus formation in the coronary arteries (19). The causative event is the burst and rupture of the atherosclerotic plaque, which reveals the blood to chromogenic lipids and triggers platelets and clotting factors. The most common coronary plaque for stenosis is a lipid-rich core with a thin fibrous cap (19).
The present study was performed to note the concentrations of the lipid profile (i.e., TC, TG, HDL, LDL, and VLDL) and cardiac profile (i.e., troponin T, troponin I, CK-MB, and CPK) in MI patients in response to the inhibition of oxidative stress. The fear of oxidative stress is increasing day by day, and the study to measure oxidative stress is least common because the patients only treat their diseases but do not see the background. The present study aimed to check and balance the causes of lethal diseases which affect human health, such as MI.
A prospective observational study by Kamal et al. showed the raised values of serum troponin I (4.90 ± 3.20) and CK-MB (17.69 ± 12.70) for risk stratum in post-MI patients (14). The aforementioned study selected 60 patients with a recent history of angina identified as Q-wave MI and non-Q-wave MI by standard echocardiography and cardiac markers within 24 hours of the onset of cardiac arrest admitted to a coronary care unit (14). The present collected the samples of those patients who were newly admitted to ICU and evaluated the serum cardiac troponin T (2006.19 pg/mL), troponin I (3011.63 pg/mL), and CK-MB (42.37 u/L) in the patients within 24 hours for further oxidative estimation.
Kedare and Singh in 2011 described that the DPPH assay is the most common and has become the first approach to assess the inhibition of oxidative stress (20). The main principle of the reaction is accompanied by the color replacement of DPPH evaluated at 517 nm, and the discoloration acts as an indicator of antioxidant efficacy (20). To keep this mechanism of action in mind, the present study also selected DPPH radical scavenging assay to inhibit the oxidative stress for MI patients. Janaszewska and Barotsz performed a DPPH assay for the first time on human plasma in 2002 (21). They collected 20 samples of healthy individuals (15 males and 5 females). All the volunteers were young, 13 of whom were smokers. The inhibition was measured at 520 nm by a spectrophotometer (21). Similarly, the current study also applied DPPH to human serum, and the current study’s sample size was highly larger, as described in the previous study. This study included the samples of 53 MI-positive patients, male and female, and inhibition was checked by a spectrophotometer at 520 nm, which showed 91.73 % inhibition in MI patients.
Mal et al. compared demographically the lipid profile of two groups in 421 participants, including the patient group (MI patients, n = 212) and the control group (non-MI patients, n = 209) (22). The TC and LDL were remarkably elevated in patients with MI. Nonetheless, HDL was lower in patients with MI. There was no remarkable difference between TG in both the control and patient groups (22). However, in the current study, the samples of positive MI patients were collected based on the non-random sampling technique, and the values were confirmed by normal ranges as provided according to the protocol. The TC (228.68 mg/dL) and LDL (119.05 mg/dL) were remarkably elevated, and HDL (33.67 mg/dL) was lower in patients with MI. It was observed that TG values (187 mg/dL) were near the borderline in MI patients. Therefore, according to this scientific research study, lipid peroxidation is a life-threatening marker for the destruction of the myocardial membrane, which was positively proved by the high level of cardiac profile and lipid profile.
5.1. Conclusions
The current study mainly investigated the role of oxidative stress in MI patients based on the variation in biochemical parameters. According to the obtained results, ROS might be the causative agent of MI with elevated levels of cardiac profile and lipid profile. Therefore, it is concluded that if an antioxidant medication is used as administrative content for MI patients, it would be helpful for the treatment of MI patients. Additionally, the present study would help the researchers to perform further research on oxidative stress and be useful for pharmaceutical purposes.