1. Context
Arsenic is found in most metals. It is mainly present in the form of sulfides in metals. Arsenic causes health problems in humans. Several million people worldwide are exposed to arsenic and its metabolites (1). Arsenic can enter the body in several ways, including drinking water, food (mainly seafood), and inhalation (2-4). Exposure to arsenic causes various diseases, including skin lesions, internal cancers, and cardiovascular diseases (5-7). According to the World Health Organization (WHO), the arsenic levels in drinking water should not exceed 10 μg/L (8). Diabetes is a metabolic disorder caused by a defect in insulin secretion (abnormal insulin secretion) and insulin function (9). There are two issues related to diabetes: (1) This disease is increasing in developing countries; and (2) type 2 diabetes (T2D) occurs at a young age (such as obese children) (10, 11). Insulin resistance, which causes abnormal insulin secretion, is a major cause of T2D mellitus (T2DM) (12, 13). Many factors increase the risk of diabetes, such as exposure to various environmental pollutants, being overweight, and lack of physical activity (14). The global prevalence of T2D is increasing; the population of people with diabetes reached more than 8% of the world’s population in 2014 (15). Diabetes is directly related to obesity, smoking, sedentary lifestyle, and low socioeconomic status (SES) (16). Socioeconomic status is recognized as an important factor in a range of health consequences (17). People with high family incomes and university degrees have a higher SES; they are less likely to develop diabetes (18).
Studies have shown that in areas where arsenic exposure is high, the risks of mortality and diabetes are also high. Areas with lower arsenic also have a lower risk of developing diabetes (19). In a study conducted in Bangladesh, researchers concluded that there was a positive relationship between occupational exposure to arsenic and the prevalence of diabetes. Occupational exposure to arsenic can significantly increase the level of glycosylated hemoglobin (20).
2. Objectives
The purpose of this study was to evaluate the effect of arsenic on the prevalence of diabetes.
3. Evidence Acquisition
3.1. Eligibility Criteria and Search Strategy
Table 1 presents the query results from different databases (including Google Scholar, Springer, ScienceDirect, PubMed, and Web of Science) and search terms. The English language was used for a review of the epidemiological literature. All relevant studies published from 2000 to 2022 were identified. A total of 466 articles were retrieved from the databases.
| Term | PubMed | ScienceDirect | Springer | Web of Science | Google Scholar | Unique Results |
|---|---|---|---|---|---|---|
| Diabetes | 21 | 33 | 18 | 17 | 63 | 152 |
| Human health | 13 | 27 | 17 | 12 | 34 | 103 |
| Arsenic | 14 | 25 | 16 | 12 | 23 | 90 |
| Air pollution | 18 | 20 | 15 | 16 | 52 | 121 |
| Total | 66 | 105 | 66 | 57 | 172 | 466 |
3.2. Eligibility Criteria
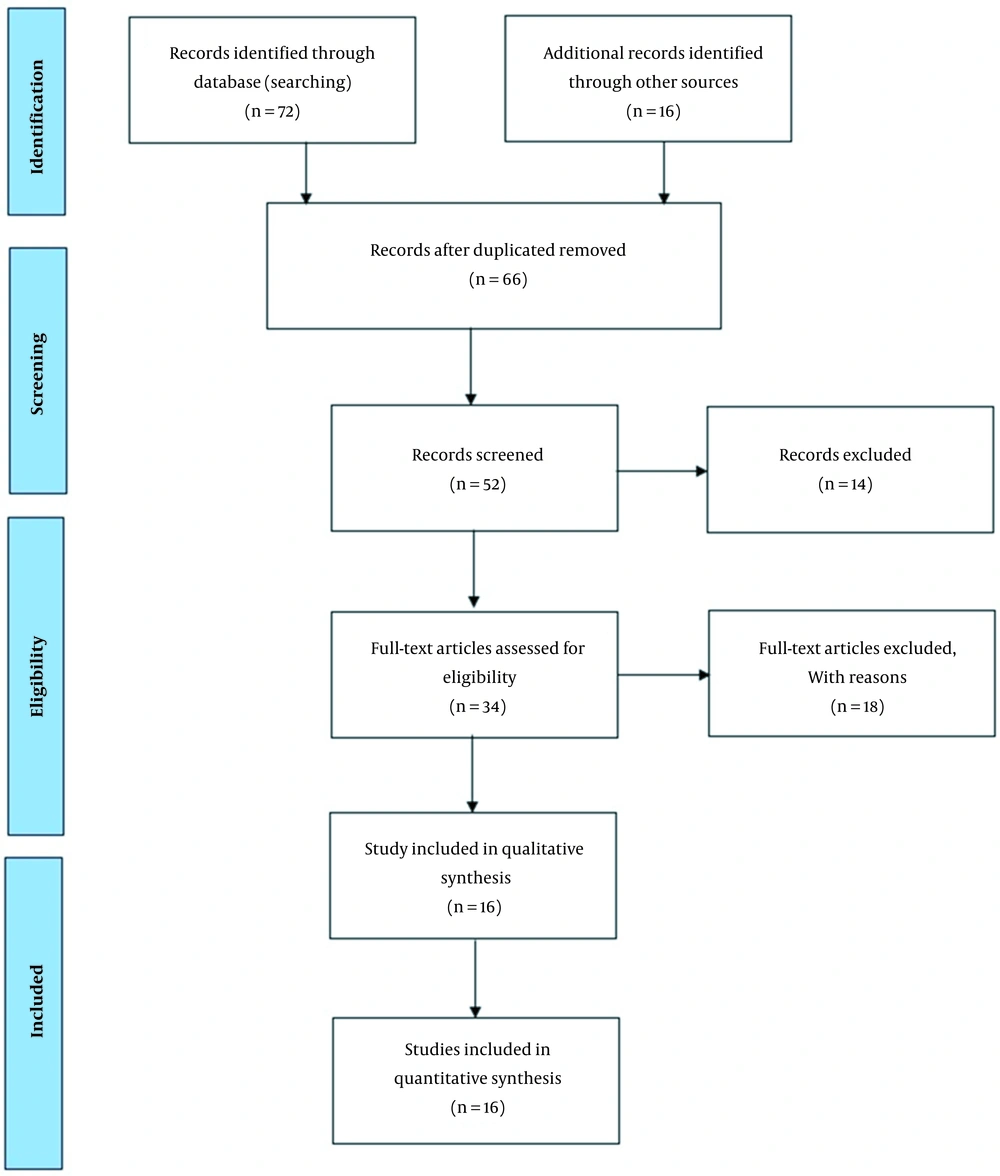
Medical Subject Headings (MeSH) used in this study were “Diabetes,” “Human Health,” “Arsenic,” and “Air pollution.” The range of 2000 to 2022 was limited in the review time efficiency of studies. First, the titles and abstracts of the extracted articles were reviewed, and those not related to the topic of our study were removed. Then, the full texts of other articles were extracted and analyzed. Eligible articles in this study included studies that focused on the effect of arsenic on diabetes and cancer. Figure 1 shows the PRISMA diagram used to extract the articles
3.3. Extraction of the Data
Data from selected publications were extracted and documented in a Microsoft Excel spreadsheet.
3.4. Ethical Approval
According to the national guidelines, individual consent is not required for studies such as this.
4. Results
4.1. Arsenic
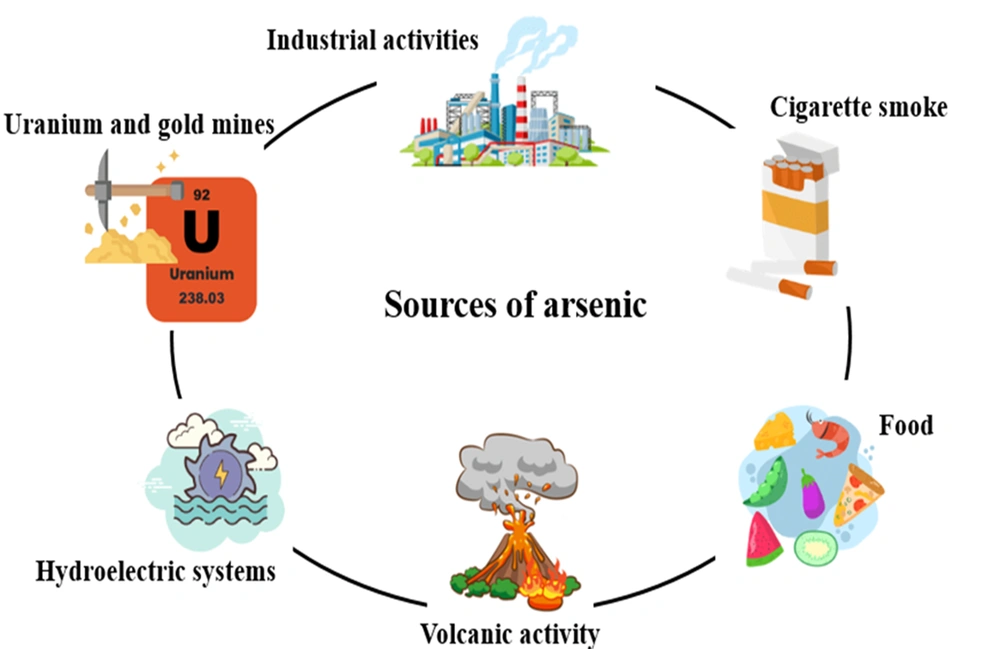
Arsenic is a natural chemical element with metallic and non-metallic properties abundantly found in the earth’s crust. This element belongs to the nitrogen family. It is widely found in sulfide ores. Arsenic is used as an insecticide due to its high toxicity. They also cause many environmental concerns, including the widespread availability of arsenic in the environment, biological toxicity, and exploitation (21). Arsenic, in terms of allotrope, exists in 3 colors (gray, yellow, and black) in nature; usually, gray color is more common than the other two colors. Gray arsenic has a very fragile structure because the bond between its layers is weak. However, it has very high stability. Its density is 5.73 g/cm3 (22, 23). Yellow arsenic has a tetrahedral structure that, despite its low density (1.97 g/cm3), has high volatility and toxicity and is an unstable allotrope. When this allotrope is exposed to sunlight, its color changes to grey (24). Black arsenic, unlike yellow arsenic, which has a soft and flexible structure, is very glassy and brittle. It is formed by cooling the steam (with a temperature of 100 to 220°C) and crystallizing amorphous arsenic (25). Arsenic is a harmful element that threatens human health. Various health organizations around the world have set guidelines for dealing with it. The immediately dangerous to life and health (IDLH) value for arsenic metal and inorganic arsenic compounds is 5 mg/m3 (5 ppb) (26). The Occupational Safety and Health Administration has set the permissible exposure limit for arsenic at 0.01 mg/m3 (0.01 ppb) (27). Arsenic has caused a lot of concern in drinking water as well. Researchers have stated that the amount of arsenic in underground water is much higher than in surface water (28). Arsenic is available from a variety of sources, including natural resources, groundwater, industrial activities, food, and tobacco (Figure 2).
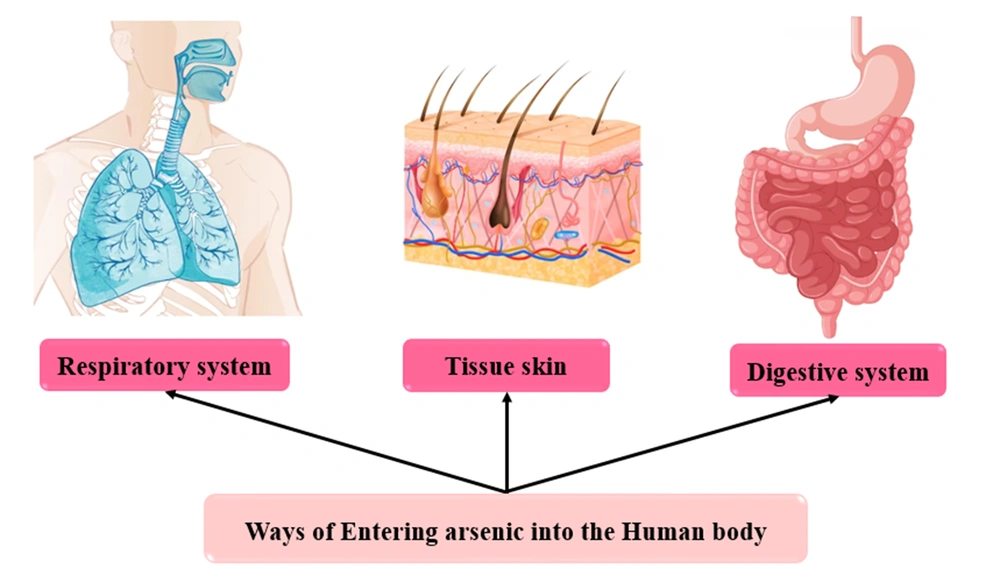
4.2. Arsenic Sources Entering the Body
4.2.1. Arsenic Entry Through Respiratory
Arsenic enters the atmosphere from both natural and anthropogenic sources. Human activities cause the release of arsenic into the atmosphere more than natural sources (approximately 1.5 times) (29). Among human activities, combustion is the most important source of arsenic production in the atmosphere (87% of total emissions). Previously, coal was used as the most important fossil fuel, containing 0.5 - 80 mg/g of arsenic (30). Burning coal releases a small amount of arsenic into the air (0.5% by weight) (31). The amount of arsenic released into the air due to the burning of fossil fuels depends on the type of fuel and the temperature of the flame. Therefore, the number of heating and cooking devices in residential and commercial places plays a key role in the amount of arsenic emission. After they are produced in thermal processes, they may be combined with other chemical elements and found as chloride or oxide. Arsenic can also stick to dust particles and spread in the air (32). Also, 2% to 3% of arsenic is released by industries for the production of lead and copper from ores. In biomass burning, some arsenic is also released, which adheres to the particles and spreads into the air (33). In the steel industry, there is a large amount of arsenic attached to the particles in the chimney outlet. Another source of arsenic emissions is the ore refining process, which produces fine particles (34). Arsenic may also be released into the atmosphere as a result of waste burning, but it should be noted that the waste must contain arsenic, which municipal waste usually contains some arsenic. Arsenic released from waste incinerators can be in gaseous or particulate forms (35). Treatment of timber can also cause the release of arsenic into the air, and researchers estimated that their release through the disposal of treated timber was 9 tons per year. It should be noted that the workers in these industries suffer from side effects due to exposure to arsenic (36). The risk assessor of the Scientific Committee on Toxicity, Environmental Toxicity and Environment (CSTEE) also confirms that exposure to the timber treatment industry can endanger people’s health (37). Table 2 shows the results of several studies on the amount of arsenic in the air.
| Author | Year | Country | City | Concentration (ng.m-3) | Reference |
|---|---|---|---|---|---|
| Aas et al. | 2005 | France | Peyrusse-Vieille | 0.2 | (38) |
| Gidhagen et al. | 1990 | Chile | Pica | 10.4 | (39) |
| Quillagua | 6.5 | ||||
| Toconao | 16.7 | ||||
| Almagro | 4.4 | ||||
| Vallenar | 3.9 | ||||
| Quillota | 30.7 | ||||
| Linares | 2.4 | ||||
| Bi et al. | 2014 | China | Shanghai | 9.4 | (40) |
| Rasmussen et al. | 2001 | Canada | Ottawa | 1.3 | (41) |
| Christoforidis and Stamatis | 2009 | Greece | Kavala’s | 27.7 | (42) |
| Zhao et al. | 2010 | China | Beijing | 18 | (43) |
| Lin et al. | 2011 | Taiwan | Mount Hehuan | 0.02-5.9 | (44) |
| Yongjie et al. | 2005 | China | Shanghai | 27 | (45) |
| Kang et al. | 2008 | S. Korea | Ulleung Island | 3 | (46) |
| Wai et al. | 2005 | Slovakia | Topoliniky | 0.44 | (29) |
| Faust et al. | 2011 | Finland | Pallas | 0.15 | (47) |
4.2.2. Arsenic Entry Through the Digestive System
Arsenic surface and underground water contamination have become a global problem. Chronic poisoning by arsenic is becoming an emerging epidemic in the Asian continent. It should be noted that millions of people worldwide are exposed to groundwater contaminated with arsenic. Arsenic in the environment is not easily destroyed. It can only be transformed into different forms or from soluble compounds to compounds with other elements (such as iron) (48, 49). According to the WHO, the European Union, some Asian countries, and the current standard of Iran for the amount of arsenic in drinking water should not exceed 0.01 mg/L (50). In recent decades, concerning the abundance of arsenic in a number of different countries that use underground water as a source of drinking water, there has been alarming information on different continents. Hundreds of millions of people, mostly in developing countries, use drinking water with arsenic concentrations several times higher than the global health guidelines (49). In 2018, Ahmad et al. tested arsenic in 3700 km2 of groundwater sources in Bangladesh, showing that 48% of the samples were above 10 µg/L (51). Arsenic enters water sources through the dissolution of minerals, discharge of industrial effluents, and through accumulation in the atmosphere. Most of the problems reported about the presence of arsenic in water supply systems are related to groundwater containing zoogenic arsenic at high concentrations. Sediments are found in the deep valley, and trivalent arsenic is its common form (52). The concentration of arsenic in water sources ranges from a few mg/L to a few µg/L. This element can act as a tracer in the saturated zone and penetrate long distances because it is not possible to prevent its movement in the water by absorption. It is noted that in areas with high geothermal activity, this element is common in water (53, 54).
4.2.3. Arsenic Entry Through the Skin
Arsenic is deposited in tissues rich in keratin (hair, nails, and skin). In Bangladesh and India, cancer of the stomach, colon, trachea, liver, bone, breast, kidney, and skin has been linked to arsenic. The skin is very sensitive to arsenic, and skin ulcers are the most common and non-malignant chronic effects of exposure to arsenic (55). Dermal absorption is considered a minor exposure route due to the low permeability of trivalent arsenate, and has not been studied in great detail so far (56). Studies in the field of occupational health have indicated that occupational exposure is the main way of skin exposure to arsenic (57). Dermal exposure appears to be relevant only where there is a risk of long-term exposure to arsenic-containing water due to lifestyle (e.g., for rice farmers in Asia) (58). Percutaneous absorption and accumulation of arsenic in the skin while working in rice fields have long been suspected of causing blackleg disease in Taiwan (59). As a result of chronic exposure to inorganic arsenic through drinking water, the symptoms of skin pigmentation (melanosis) and then hyperkeratosis appear. Cancer is the last stage of the reaction to long-term exposure to arsenic, which usually takes more than ten years for its effects to develop (60). The first case of chronic exposure to arsenic in Iran (Kurdistan Province) was known in 1986 when a woman suffered from gangrene in the leg area. In a study conducted in India in 11 villages, 989 people were screened, and 137 adults and 17 children had severe skin ulcers (61). A study conducted between 2005 and 2007 on residents living near a river in Pakistan showed that 61% - 73% of patients suffered from arsenic toxicity (60). In a study conducted in Bangladesh, it was found that of the 1481 people, 430 were suffering from keratosis and pigmentation. In this study, a significant difference was observed between exposure to arsenic and the prevalence of skin diseases (Figure 3) (5).
4.3. Side Effects of Arsenic on Human Health
Arsenic is a natural element that is toxic and dangerous to humans, animals, and the environment. They have many applications in industry, pharmacy, and agriculture. Arsenic in aquatic bodies can be a potential hazard to living organisms (62). Very low concentrations of arsenic in drinking water can have harmful effects. The new water quality law in the Environmental Protection Agency has set the permissible level of arsenic in drinking water at 10 ppb (50). Arsenic is an element that is found in nature and absorbed by plants and grains through irrigation (63). As a result, humans and animals become ill by consuming arsenic-contaminated foods. Arsenic can cause many diseases in animals, such as intestinal, pulmonary, respiratory, cardiac, and kidney disorders (64). Arsenic enters the body in a variety of ways, including inhalation, digestion, and skin. It accumulates in various glands of the body, such as the liver, lungs, kidneys, and skin (65). Arsenic contamination has a wide range of medical symptoms (66). Arsenic exposure can be difficult to diagnose because it can be found in many other diseases (67). In industrial areas, arsenic production is higher than in non-industrial areas, and air is the main source of arsenic poisoning. Arsenic in the air can be a risk factor for human and animal health (68). Arsenic enters the body of animals in different ways, such as contaminated drinking water, grasslands, vegetables, and various leaves. Arsenic can cause poisoning in animals and humans. Biomarkers of arsenic exposure in humans and animals include urine, blood, hair, and milk (69).
4.4. Arsenic Toxicity (Carcinogenicity)
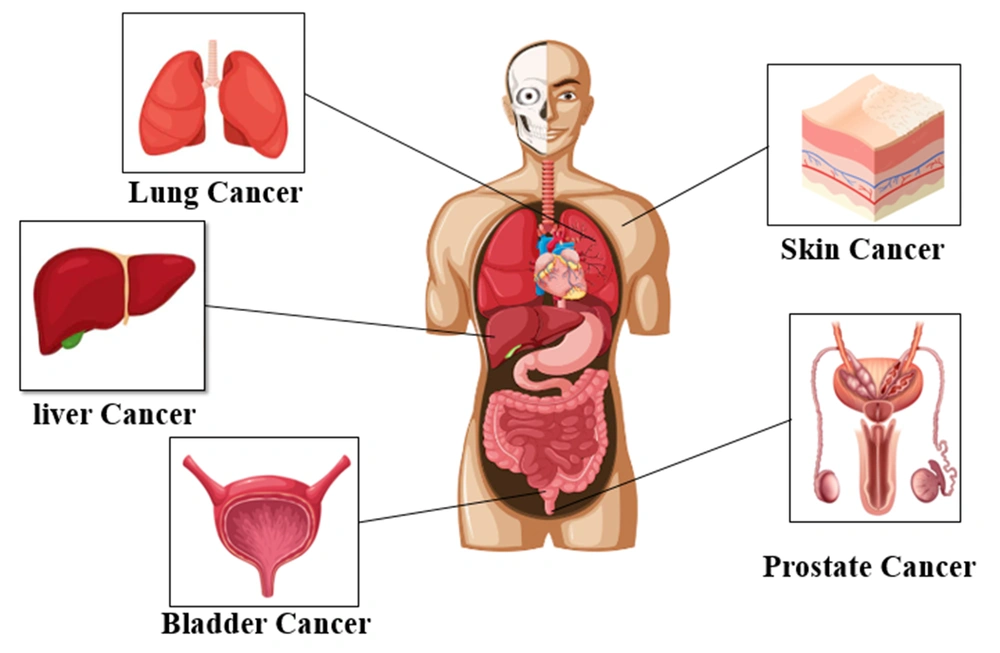
Arsenic is a carcinogen that causes cancer both in the respiratory and gastrointestinal tracts (70). In the 1980s, the International Agency for Research on Cancer classified arsenic as a carcinogen (71). Studies in the United States have confirmed that excessive exposure to arsenic can cause cancer (65). Arsenic can be classified as both group 1 and group 2 carcinogens (group 2B). Epidemiological data show that arsenic can enter the body through digestion, inhalation, and skin and cause cancerous (lung, skin, and bladder) and non-cancerous disorders (Figure 4) (72).
4.5. Skin Cancer
The association between arsenic exposure and skin cancer has been proven. In particular, trivalent arsenic causes skin cancer (73). Contact with arsenic often causes basal cell carcinoma or keratinized squamous cell carcinoma. For example, a cohort study in Taiwan found that the incidence of arsenic-related skin cancer was 150 per 10,000 people per year. The incidence of skin cancer with arsenic depends on several factors, including the duration of drinking water use, the concentration of arsenic in drinking water, and exposure to arsenic accumulation (74). Researchers in several European countries concluded that even low exposure to arsenic can cause skin disorders and cancer.
4.6. Lung Cancer
Arsenic can cause the most damage to the lungs. In fact, the most well-known cancer that is involved in exposure to arsenic is lung cancer. Numerous studies have examined the association between arsenic and lung cancer. Epidemiological studies have shown that the possibility of lung cancer is much higher in people who are more exposed to arsenic than in people who are less exposed to it. A study conducted in Taiwan showed a high correlation between mortality from lung cancer and drinking water consumption (with high concentrations of arsenic) (75). In addition, reducing the concentration of arsenic in tap water reduces lung cancer. However, people who smoke are more likely to be exposed to arsenic. As a result, smokers develop lung cancer due to their high arsenic intake (76).
4.7. Bladder Cancer
Studies in Taiwan and Bangladesh have confirmed that exposure to arsenic plays a key role in bladder cancer. Long-term exposure to arsenic is very effective in causing urinary tract (bladder) disorders. However, there is no association with low exposure to arsenic (less than 100 μg/L). Epidemiological studies have shown that very low exposure to arsenic cannot hurt bladder function. The inability to accurately measure the levels of exposure to cigarettes and arsenic is a major limitation of these studies.
4.8. Liver Cancer
There is debate as to whether arsenic can cause liver cancer. Studies in some countries, including Argentina, Chile, and Denmark, have shown a link between arsenic consumption and liver cancer, but due to limited access to representative data, no theoretical agreement has been reached (77, 78). In a cohort study conducted in Taiwan, researchers concluded that there was a strong association between long-term exposure to arsenic and liver disorders. At the same time, at lower concentrations, this value showed little association (79).
4.9. Prostate Cancer
Studies have shown an association between high concentrations of arsenic and prostate cancer (80). There is little association between low arsenic concentrations and prostate cancer. However, a study in the United States (3932 natives) confirmed an association between exposure to high arsenic concentrations and prostate cancer (81).
4.10. Non-carcinogenic Effects
4.10.1. Effects on Brain Function
Arsenic has a cumulative property and can accumulate in the body. Behavioral and neurological disorders during puberty and neurobehavioral changes in adulthood can be due to arsenic accumulation in childhood (82). In addition, these effects can be due to the accumulation of lead in the body. Therefore, caution is necessary: Neuritis is one of the most well-known effects of arsenic on the human body and affects the sensory functions of peripheral nerves. The relationship between arsenic exposure and neurological disorders has been proven in many countries (83).
4.11. Diabetes
A recent debate has focused on the link between arsenic exposure and diabetes. In particular, obese people over the age of 40 who are exposed to mineral arsenic are more likely to develop T2D. The incidence of diabetes in regional residents in Taiwan was 2 to 5 times higher than those exposed to high arsenic drinking water levels, indicating that arsenic may affect the incidence of diabetes (84, 85). Previous research has established an association between consuming high levels of inorganic arsenic in drinking water and T2D, but little is known about the effect of low levels of arsenic (86). Figure 5 shows the non-carcinogenic effects of arsenic on human health.
4.12. Epidemiological Studies of Diabetes
Pancreatic islet β cells play a key role in insulin production and secretion. The pathophysiological processes of type 1 and type 2 diabetes can be due to insulin deficiency (87). It should be noted that insulin secretion is due to the transfer of glucose to pancreatic β cells. As glucose enters the cell through a transmitter, glycolysis begins, and adenosine triphosphate is eventually produced. The adenosine triphosphate-sensitive potassium channel closes and depolarizes voltage-dependent calcium. The channel in the cell membrane eventually causes insulin exocytosis of β cells (88, 89). On the other hand, dysfunction of pancreatic β cells causes diabetes. This disorder can be due to oxidative stress. Oxidative stress can be caused in a number of ways, such as the electron transfer chain in the mitochondria and the non-enzymatic glycosylation reaction. Pancreatic β cells are among the most vulnerable cells to oxidative stress (90).
Diabetes is a common disease in the world that affects many people. This disease is caused by a long-term increase in blood sugar levels (91). Diabetes causes many short- and long-term symptoms in the body. Short-term symptoms of diabetes include frequent urination, increased thirst, hunger, diabetic ketoacidosis (DKA), and hyperosmolar coma (92). Long-term symptoms of diabetes include kidney failure, diabetic heart disease, stroke, leg ulcers, and impaired vision. Diabetes occurs for two reasons: (1) Insufficient production of insulin by pancreatic cells; and (2) lack of proper response of body cells to insulin production (93). There are three types of diabetes: T1D, T2D, and gestational diabetes.
Type 1 diabetes, which is mostly seen in teenagers, is an autoimmune reaction where the body mistakenly attacks its own cells. This reaction prevents the body from making insulin. This type of diabetes is known as juvenile diabetes. Type 1 diabetes is seen in 5% - 10% of diabetic people. The way to treat it is to inject insulin daily.
In T2D, the body’s cells do not respond properly to insulin. In addition, insulin deficiency may occur. The disease is also called non-insulin-dependent diabetes mellitus (NIDDM) or adult-onset diabetes. Excess body weight and lack of physical activity are the causes of this type of diabetes (94). Gestational diabetes occurs when a woman’s blood glucose level rises without a previous history of diabetes. There are several ways to prevent diabetes, such as a healthy diet, exercise, avoiding smoking, and maintaining a normal weight. There are several treatments for diabetes: The injection of insulin in T1D patients (95) and treatment of T2D with or without insulin (94). Gestational diabetes resolves after childbirth (96).
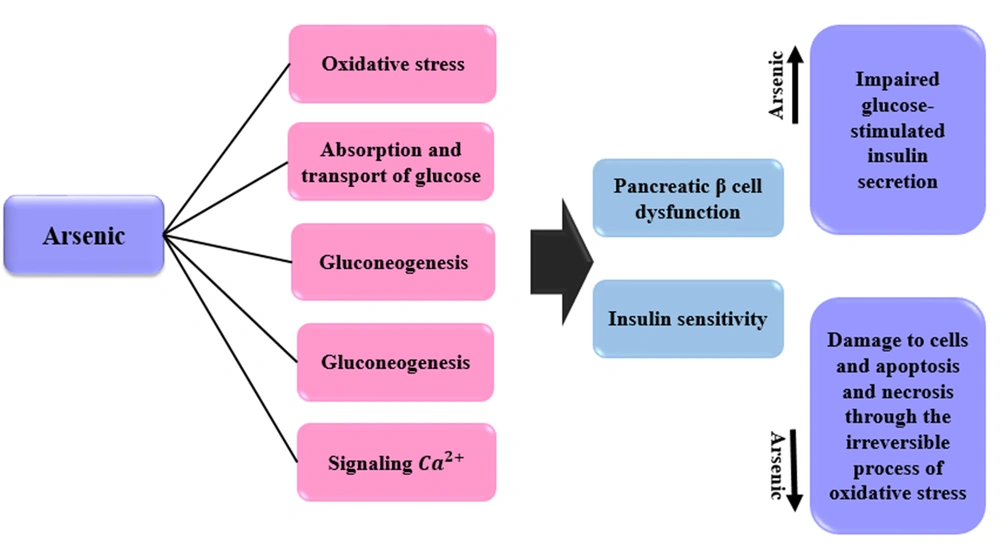
4.13. Mechanisms of the Effects of Arsenic on Diabetes
Arsenic can affect pancreatic β cell function and insulin sensitivity in a number of ways, including oxidative stress, glucose uptake and transport, gluconeogenesis, fat cell differentiation, and Ca2+ signaling (97). In general, three epidemiological studies have been performed on the association between arsenic and diabetes: (1) A study that examined stress responses in different cell types: This study was performed with a high concentration of arsenic (≥ 1 mM). This concentration led to cytotoxicity; (2) studies that evaluated the inhibition of insulin signaling and the uptake of insulin-dependent glucose by adipocytes or myotubes. Arsenic concentration was low in these studies (< 100 μM) (98, 99); (3) studies that have been conducted on isolated insulinoma or pancreatic islet cell lines. These studies have shown that arsenic affects β cells to inhibit the expression and/or secretion of insulin (100). Relatively low concentrations of arsenic lead to impaired glucose-stimulated insulin secretion (100, 101). Irreversible damage (including oxidative damage) to β cells and apoptosis/necrosis occurs at high concentrations of arsenic (102). Inorganic arsenic plays an important role in the dysfunction of β cells in diabetes through oxidative damage (103). Oxidative stress contributes to arsenic toxicity. Inorganic arsenic and its methyl trivalent metabolites are associated with oxidative stress (104). In T2D, whole-body glucose homeostasis is disrupted due to insulin resistance. This type of diabetes also interferes with the use of glucose by peripheral tissues. Arsenite and/or methyl trivalent metabolites induce insulin resistance in adipocytes (99, 105). Mechanisms of the effects of arsenic on diabetes are expressed in Figure 6.
5. Conclusions
According to the literature, arsenic is available in a variety of sources, including natural resources, groundwater, industrial activities, food, and tobacco. Potential routes of arsenic exposure in humans include drinking water, industrial manufacturing, smoking, diet, cosmetics, and air. Arsenic can cause health problems, such as skin cancer, lung cancer, liver cancer, prostate cancer, and diabetes. Diabetes is a common disease in the world that affects many people. This disease is caused by a long-term increase in blood sugar levels. There are three types of diabetes: T1D, T2D, and gestational diabetes. In T1D, enough insulin is not produced. In T2D, the body’s cells do not respond properly to insulin. Excess body weight and lack of physical activity are the causes of this type of diabetes. Gestational diabetes occurs when a woman’s blood glucose level rises without a previous history of diabetes. Arsenic can affect pancreatic β cell function and insulin sensitivity in a number of ways, including oxidative stress, glucose uptake and transport, gluconeogenesis, fat cell differentiation, and Ca2+ signaling.