1. Background
Supply chain management in competitive environments is crucial for organizations to achieve their goals effectively. The complexity of the supply chain presents significant challenges in its management (1). Recently, with the growth of the health and medical products market, supply chain management has become increasingly important in this sector (2). Medicine plays a vital role in public health, and even minor disruptions in the pharmaceutical supply and distribution chain can lead to societal crises (3). The absence of medicine can threaten human lives. Moreover, as medicines have a finite shelf life, their value diminishes as they approach their expiration date. Consequently, companies sometimes sell drugs under special conditions, influencing the pharmaceutical supply and distribution chain (4).
The primary objective of designing a supply chain and maintaining its effectiveness is to meet consumer demands, remain competitive in the market, and achieve adequate profits (5). The pharmaceutical industry faces numerous challenges, including rising costs that correlate with improvements in the quality of pharmaceutical products. Key challenges in drug manufacturing include assessing the potential value of drugs, evaluating their impact on health, expanding capacity for new facilities, and planning production (6). The need to meet consumer expectations and manage escalating costs compels supply chains to seek ways to enhance service quality (7).
Challenges also arise from sanctions and the consequent financial difficulties within the health system. Thus, cost management, control, and revenue enhancement are particularly crucial in the pharmaceutical sector, which bears a significant cost burden. Reforming pharmaceutical policies in developing countries is a vital aspect of health and treatment sector reforms. The critical role of medicine in patient treatment processes and enhancing public health is widely recognized. Consequently, there is a clear need to establish a scientific and systematic supply chain (8).
Several studies have explored the factors and challenges affecting the drug supply chain. Olutuase et al. explored the challenges faced by Nigeria in the supply of medicines and vaccines. Their findings highlighted significant issues such as inadequate storage infrastructure, financial constraints, transportation difficulties, insufficient human resources, and weak or poorly implemented policies (9). Tegegn et al. assessed the challenges and opportunities of clinical pharmacy services in Ethiopia from healthcare practitioners' perspectives. They identified several challenges, including inadequate promotion of pharmaceutical services, discontinuity of clinical pharmacy services in hospital departments, poor pharmaceutical information services, lack of organizational commitment among staff, and conflicts of interest in the drug supply chain (10). Shahbahrami et al. concluded that poor pharmacy liquidity management, ineffective interactions between medical supply managers and pharmacy staff, and unnecessary drug prescriptions are among the issues affecting the drug supply chain (11).
The drug supply chain is an integral part of the healthcare system. Without sufficient attention, the concept of health cannot progress within society.
2. Objectives
Given the significance of managing the pharmaceutical supply chain, this study categorized the key variables related to it using a systematic review approach.
3. Methods
3.1. Protocol
This study evaluated all research related to supply chain management in hospital settings to identify factors impacting the drug supply chain through a systematic review. The review adhered to a predefined protocol for systematic reviews, meta-analyses, and PRISMA guidelines (12). This protocol is registered in the International Registry of Systematic Reviews (PROSPERO 2023, CRD42023429776), and the published methodology is open for public comment at the following link: https://www.crd.york.ac.uk/PROSPERO.
3.2. Search Strategy
The search strategy was executed across international and national databases from January 30, 2000, to March 30, 2023. Articles were identified through an electronic search, and their bibliographies were further reviewed. The search included international databases such as PubMed, Web of Science, Scopus, Google Scholar, Science Direct, Springer, and ProQuest, as well as national databases including SID, MAGIRAN, IRANDOC, and IranMedex. Keywords pertaining to the topic, such as "supply chain," "medical service supply chain," "drug supply and distribution chain," "drug supply chain management in hospitals," "drug supply and distribution chain in medical centers," among others, were employed in the search.
3.3. Study Inclusion and Exclusion Criteria
The inclusion criteria encompassed all relevant studies published between January 30, 2000, and March 30, 2023. Letters to the editor and editorials were excluded due to potential shortcomings in comprehensiveness and data quality. Additionally, articles not written in Persian or English were omitted from the study.
3.4. Selection of Studies
Following the initial search and review of article titles and abstracts, the full texts of relevant articles were selected for further reading and subsequently analyzed according to the research objectives. The selection process involved multiple steps by three researchers. Initially, irrelevant titles were discarded after reviewing titles and abstracts. Further scrutiny of the abstracts led to the exclusion of additional studies. Books, conference articles, reports, and newspaper editorials were also excluded from the analysis. Articles not in Persian or English were likewise excluded. All remaining articles were evaluated in detail.
3.5. Database Search
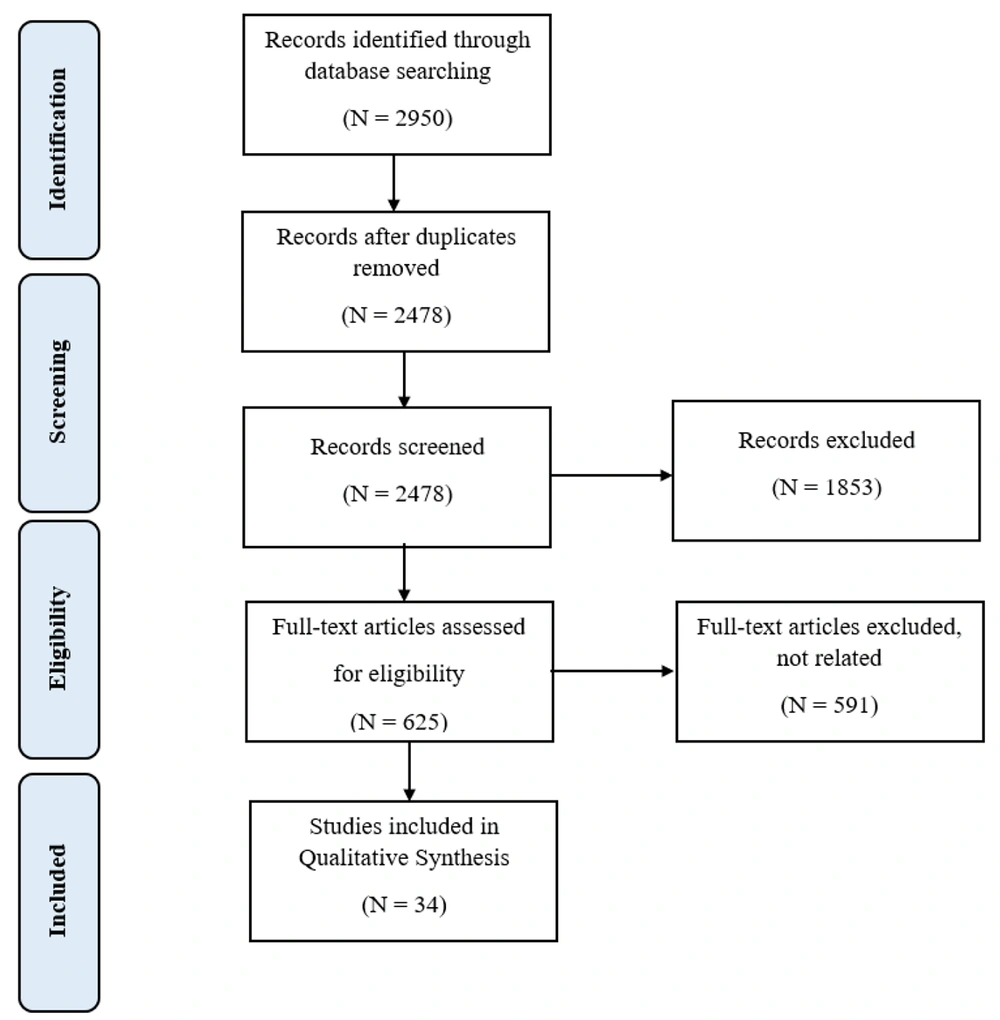
An initial search across various databases yielded 2 950 articles (980 from PubMed, 500 from Web of Science, 360 from Scopus, 700 from Google Scholar, and the rest from national databases). Of these, the full texts of 625 articles were considered for further reading based on the preliminary title and abstract review, most of which were sourced from the PubMed database. Ultimately, 34 articles underwent qualitative review. The quality of these articles was assessed using the CASP checklist, starting with an evaluation of the articles’ objectives; if deemed appropriate, the checklist was applied (Figure 1).
3.6. Qualitative Assessment
The quality of the articles was assessed using the CASP (Critical Appraisal Skills Program) checklist. Initially, the objectives of each article were evaluated, and if found appropriate, the CASP checklist was then applied. This checklist comprises 10 questions and facilitates a structured review. Articles were categorized into three quality levels based on their scores: Studies scoring less than 4 were considered low quality, those scoring between 4 and 7 were deemed medium quality, and scores of 7 or higher indicated high quality. To enhance credibility and minimize review bias, the quality assessment was independently conducted by three reviewers. Discrepancies among the reviewers were resolved through discussion and consensus (13).
4. Results
After the evaluation of the final 34 studies, key variables related to the supply chain and distribution of pharmaceutical items were summarized. According to the CASP checklist, all reviewed articles achieved satisfactory quality scores, as presented in Table 1.
| Authors | Year | Type of Study | Country | Study Location | General Topic | |
|---|---|---|---|---|---|---|
| 1 | George and Elrashid (14) | 2023 | Analytical | Bahrain | Hospital pharmacies | Inventory management and pharmaceutical supply chain performance |
| 2 | Martinez et al. (15) | 2023 | Retrospective review | Sweden | A university hospital | Examination of the protein drug supply chain |
| 3 | Olutuase et al. (9) | 2022 | Review | Nigeria | Hospitals | Medicines and vaccines supply chains challenges |
| 4 | Hu et al. (16) | 2022 | Analytical | Pakistan | Pharmaceutical companies | The influence of knowledge management capacities on pharmaceutical firms’ competitive advantage |
| 5 | Nasiripour et al. (17) | 2022 | Qualitative quantitative | Iran | Hospitals | Factors affecting hospital services supply chain |
| 6 | Vargas et al. (18) | 2021 | Qualitative | Mexico | Government hospitals | The implementation of pharmaceutical services in public hospitals |
| 7 | Hamma-Adama and Labaran (19) | 2021 | Qualitative | Nigeria | Pharmaceutical companies | The Nigerian pharmaceutical supply chain |
| 8 | Bastani et al. (20) | 2021 | Qualitative | Iran | Food and drug deputy | Strategies to improve pharmaceutical supply chain resilience |
| 9 | Haial et al. (21) | 2021 | Delphi | Morocco | Pharmaceutical companies | Transportation-strategy decision-making process for a supply chain |
| 10 | Haji et al. (22) | 2021 | Review | Qatar | Pharmaceutical companies | Traceability technologies for establishing a safe pharmaceutical supply chain |
| 11 | Tirivangani et al. (23) | 2021 | Qualitative | Namibia | pharmaceutical companies | Impact of COVID-19 pandemic on pharmaceutical systems |
| 12 | Hambisa et al. (24) | 2020 | Analytical | Ethiopia | Hospitals | Attitudes, opportunities, and challenges for clinical pharmacy services |
| 13 | Jbaily et al. (25) | 2020 | Analytical | Ethiopia | Health system | Insights from mathematical modeling of drug supply chains |
| 14 | Carvalho et al. (26) | 2020 | Review | Brazil | Hospitals | Effectiveness of the automated drug dispensing system |
| 15 | Shahbahrami et al. (11) | 2020 | Cross-sectional descriptive | Iran | Hospital pharmacies | Determinants of drug sustainable supply chain management |
| 16 | Vledder et al. (27) | 2019 | Randomized trial | Zambia | Clinical centers | Improving supply chain for essential drugs in low-income countries |
| 17 | Silva and Mattos (28) | 2019 | Qualitative | Brazil | Hospitals | Critical success factors of a drug traceability system |
| 18 | Tegegn et al. (10) | 2018 | Qualitative | Ethiopia | Gondar University Hospital | Challenges and opportunities of clinical pharmacy services |
| 19 | Iqbal et al. (29) | 2017 | Review | India | Indian hospitals | Medicines management in hospitals |
| 20 | Seidman and Atun (30) | 2017 | Review | United States | Hospitals | Drug supply chain, cost savings and improved access |
| 21 | Mokheseng et al. (31) | 2017 | Analytical | South Africa | Manapo Hospital | Supply chain solutions to improve the distribution of antiretroviral drugs to clinics |
| 22 | Esmaeillou et al. (32) | 2017 | Qualitative and analytical | Iran | Pharmaceutical companies | Factors affecting the pharmaceutical supply chain management |
| 23 | Brako et al. (33) | 2016 | Quantitative | Ghana | Pharmaceutical companies | Investigating the risks in the pharmaceutical supply chain |
| 24 | Awad et al. (34) | 2016 | Analytical | Jordan | Food and Drug Administration | Analysis of the causes of drug shortages |
| 25 | Holm et al. (35) | 2015 | Analytical | Haiti | Hospital | Medication supply chain management |
| 26 | Jaberidoost et al. (36) | 2015 | Review and qualitative | Iran | Pharmaceutical companies | Pharmaceutical supply chain risk assessment |
| 27 | Mensah et al. (37) | 2015 | Analytical | Ghana | Government hospitals | Optimizing drug supply chain in hospital pharmacy department |
| 28 | Hetzel et al. (38) | 2014 | Analytical | Papua New Guinea | Clinical centers | Quality of antimalarial drugs and antibiotics |
| 29 | Karimi et al. (39) | 2014 | Descriptive analytical | Iran | Hospitals | Factors affecting drug pert of experts in hospitals |
| 30 | Jaberidoost et al. (40) | 2013 | Review | Iran | Pharmaceutical companies | Pharmaceutical supply chain risks |
| 31 | Yu et al. (41) | 2010 | Review | China | Health care system | Issues of the pharmaceutical supply chain |
| 32 | Bedouch et al. (42) | 2008 | Analytical | France | University Hospitals | Drug supply chain safety in hospitals |
| 33 | Cohen et al. (43) | 2005 | Interventional | United States | Hospitals | Medication safety program reduces adverse drug events |
| 34 | Krampera et al. (44) | 2004 | Analytical | Italy | Hospitals | Computer‐based drug management in a bone marrow transplant unit |
Table 2 categorizes the key variables associated with the pharmaceutical supply chain. These variables are divided into five categories: Monitoring and control (16 variables), information technology and intelligence (4 variables), human capital (10 variables), physical and financial resources (16 variables), and suppliers (4 variables).
| Main Factors | Key Variables |
|---|---|
| Monitoring and control | Continuous monitoring, control, and management of the inventory of medicinal items in the hospital with an effective and efficient system. |
| Support and continuous support of hospital management | |
| Compilation of effective regulations and transparency of the legal framework | |
| Development of applicable regulatory and legal processes | |
| Supervision of drug warehouse, drug stock of wards, and hospital pharmacy | |
| The process of controlling and documenting the circulation of drugs in the hospital's drug warehouse | |
| Supervision of the pharmaceutical committee of the hospital in the preparation, distribution, storage, and consumption of medicines | |
| Attention to crisis management and emergencies in the drug supply chain | |
| The need to monitor and manage commonly used drugs in critical situations | |
| Control of conflicts of interest in the drug supply and distribution chain | |
| Establishing a balance between the supply and demand of pharmaceutical items in the drug supply chain | |
| The necessity of specialization in drug supply chain management | |
| Reducing bureaucratic bottlenecks in the supply and distribution chain of drug logistics | |
| Improving the pharmaceutical service system in the drug supply chain | |
| Preventing induced demand in the provision of unnecessary pharmaceutical services | |
| The need for hospital flexibility and agility against changes in the drug supply chain | |
| Information technology and intelligence | Redesigning the drug supply chain system using accurate technical evidence to prevent drug shortages in the healthcare system |
| Awareness of block chain technology among pharmaceutical supply chain stakeholders to create safe and sustainable conditions with drug traceability | |
| Smartening the drug supply chain and distribution system to predict the demand for drug items according to the performance of the hospital | |
| Creating the necessary conditions for modern storage in hospitals with up-to-date technologies | |
| Human capital | Training and empowering human resources involved in the drug supply chain |
| Training of human resources regarding the accurate registration of medicinal items in the system to prevent the wastage of medicinal products in the hospital, emphasizing organizational culture. | |
| Training hospital residents to prevent unnecessary prescription of drugs and indiscriminate use of medicinal items, emphasizing organizational culture. | |
| Ensuring the employment of expert managers to manage the costs of pharmaceutical items | |
| Ensuring a sufficient number of personnel involved in the drug supply chain to avoid delays in drug distribution and delivery | |
| Necessity of interaction between the technical manager of medical supplies and hospital pharmacy staff | |
| Ensuring the existence of specified responsibilities and roles for employees involved in the supply chain and distribution of drugs in the hospital | |
| Planning to strengthen organizational commitment by considering sufficient incentives among employees involved in the supply chain and distribution of pharmaceutical items. | |
| Promoting knowledge management among employees involved in the drug supply chain | |
| Compliance with the professional ethics of employees in the drug supply chain | |
| Physical and financial resources | Creating suitable physical conditions in the warehouse of pharmaceutical items, including maintaining the appropriate temperature and humidity and preventing environmental pollution and shock caused by impact, vibration, and exposure to light. |
| Creating sufficient physical space and proper infrastructure in the condition of availability of excessive amounts of drugs in hospital drug stores | |
| Observance of stable safety in hospital drug warehouses | |
| Creating suitable conditions for the transportation of medicinal items | |
| Appropriate and practical design of the drug supply and distribution network with an emphasis on outsourcing the process of transporting pharmaceutical items | |
| Creating a support system and drug procurement electronically | |
| Increasing the number of servers inside the facility and improving the quality of the hospital's support system to prevent the late distribution of medicinal items. | |
| Finding the root causes of inefficiency in the drug supply and distribution chain with more emphasis on the support processes and procurement of pharmaceutical items. | |
| Attention to cash management in hospital pharmacies | |
| attention to financial resources and resource allocation methods in the drug supply chain | |
| Choosing the methods of drug purchase, either centralized or semi-centralized purchasing, with an emphasis on strategic purchasing based on national policies | |
| Outsourcing hospital drug supply services | |
| Developing a new and efficient drug pricing mechanism | |
| Effective monitoring and control of the drug pricing process | |
| Creating suitable conditions for rational pricing | |
| Designing a competitive business model for the optimal price and quality of services | |
| Drug suppliers | The necessity of loyalty and commitment of suppliers of pharmaceutical items to hospitals |
| The need to respond to suppliers of medicinal items in emergencies | |
| Flexibility of suppliers with emphasis on the financial strength and liquidity in the supply chain and distribution of pharmaceutical items | |
| The external relations of the hospital with the suppliers of pharmaceutical items |
5. Discussion
In this study, critical variables related to the drug supply chain were identified using a systematic review approach.
5.1. Monitoring and Control
Effective management and continuous monitoring of drug inventory in hospitals are crucial to the drug supply chain. Inadequate inventory control can negatively impact patient health. The efficiency of hospital supply chain performance is vital for increasing patient satisfaction (45). The primary goals of managing, controlling, and monitoring drug inventory in hospitals are to ensure drug availability and reduce costs. Odhiambo and Kihara explored the impact of inventory management practices on supply chain performance in public health facilities in Kenya. Their findings indicated that lean inventory practices, accurate inventory records, and information technology significantly influence supply chain performance (46). Hospital management also plays a critical role in providing continuous support. Barati et al. examined the challenges faced by hospital management in Shiraz hospitals and found that a lack of support from senior managers to hospital staff is a major challenge (47). This lack of support is similarly detrimental to clinical pharmacy services, which struggle to achieve their goals without adequate management backing (48). Additionally, developing enforceable regulatory and legal processes is crucial. Monitoring the drug distribution system helps maintain integrity throughout the supply chain (49). Another significant aspect of management and oversight within the drug supply chain concerns monitoring drug warehouses. Studies have shown that adverse effects from drug prescriptions, often due to incorrect prescriptions and inadequate monitoring of drug distribution in hospital departments, can double the length of patient stays (50).
5.2. Information Technology and Intelligence
Emphasizing new technologies and knowledge within the drug supply chain is crucial. Nasiripour et al. recognized technology as a significant influence on the supply chain of hospital services (17). Visconti and Morea noted that digital healthcare drives innovation, growth, and competition, highlighting that 21st-century medicine increasingly relies on technology (51). Nagariya et al. identified technology as the backbone of hospital service supply chains (52). Another essential aspect is the "smartization" of the drug supply chain and distribution system, which involves predicting pharmaceutical demand based on hospital performance. George and Elrashid found that demand forecasting positively impacts drug supply chain performance (14). Furthermore, Polater and Demirdogen emphasized the importance of using the latest technologies to predict pharmaceutical demand (53).
5.3. Human Capital
Training and empowering human resources within the drug supply chain is crucial. It is vital to train and empower all treatment staff, particularly hospital pharmacy personnel, to minimize medication errors (54). Empowering employees is a highly effective strategy for development, prosperity, and enhancing productivity in hospitals. Various empowerment methods at the start of employment can boost both organizational and individual productivity. Empowering pharmaceutical personnel can significantly improve supply chain performance (55). Ensuring an adequate number of staff in the drug supply chain to avoid delays in drug distribution and delivery is another important factor. Hambisa et al. assessed clinical pharmacy services in Ethiopia and found that labor shortages and insufficient supervision were significant challenges (24). These findings corroborate those of other studies (48, 56). Hospital management should focus on creating opportunities to recruit more healthcare providers and train professionals to improve health services. Additionally, planning to enhance organizational commitment by offering adequate incentives to employees involved in the supply and distribution of pharmaceutical items is crucial. Organizational commitment is a key motivational factor widely discussed in organizational studies today (57).
5.4. Physical and Financial Resources
Maintaining proper physical conditions in the warehouse for pharmaceutical items is crucial, including optimal temperature and humidity, as well as protecting against environmental pollution, shock from impacts, vibrations, and exposure to light. The general warehouse plays a key role in hospitals, tasked with timely supplying various departments by storing the hospital’s materials, resources, and consumables. It is imperative to manage the inventory of medical goods, particularly pharmaceutical items, due to their impact on patient health. Necessary measures and plans should be implemented to ensure suitable physical conditions in hospital supply warehouses, including those for pharmaceuticals, such as maintaining appropriate temperature and humidity levels (58). Another critical aspect is having sufficient physical space and adequate infrastructure to store excess drugs in hospital drug warehouses. Inadequate physical space in pharmaceutical warehouses can lead to significant issues with staff movement, drug storage, and inspection. Additionally, drug accumulation on shelves can cause irreparable damage to the hospital’s medicine warehouse (24).
5.5. Drug Suppliers
The ability to respond quickly to suppliers of pharmaceutical items in emergencies is a crucial factor in the drug supply chain. Effective emergency response to accidents and disasters is necessary to provide high-quality services. Planning for accidents and disasters is essential in all hospitals, and the critical role of hospitals during crises should be prioritized in planning and budgeting (59). Effective external communication with pharmaceutical suppliers is also important. Hospital managers play a vital role in leading and supervising both clinical and administrative personnel to enhance efficiency across different departments. It is a manager's duty to establish effective communication between various hospital departments. Considering the personal characteristics of the manager, alongside technical and organizational factors, is crucial for achieving organizational goals (60).
5.6. Limitations
Most of the studies included in this review are geographically concentrated in developing countries, which may introduce bias into the data due to their significant contribution to the findings. Additionally, the relatively small number of studies from advanced countries complicates geographic comparisons. Future research should address this issue to provide a more balanced perspective.
5.7. Conclusions
Regulating the drug supply chain is crucial for the health system. Disruptions in this chain can lead to crises for patients. The findings of this review identify critical factors in the drug supply chain, including Monitoring and Control, Information Technology and Intelligence, Human Capital, Physical and Financial Resources, and Drug Suppliers. Therefore, policymakers and healthcare managers should give special consideration to these factors, especially the managerial and regulatory aspects, to mitigate challenges in drug supply through effective strategies.