1. Background
Today, despite the use of various methods to control hospital infections, bacterial resistance against antimicrobial agents continues to affect sensitive and vulnerable patients in various hospital units (1). Consequently, maintaining healthcare standards in the hospital environment is crucial for infection control (2). A significant factor in the spread of hospital infections is the improper use of disinfectants. Employing effective and safe disinfectant solutions with minimal damage to equipment and personnel is fundamental to disinfection practices (3). Unfortunately, inappropriate choices of disinfectants, unsuitable environmental conditions, lack of training, and personnel negligence have often reduced the effectiveness of disinfectants, leading to inadequate removal of environmental pathogens and increasing the likelihood of hospital infections (4).
Understanding the ways infections are transmitted can help break this cycle. The five main transmission methods of microorganisms are contact, suspended particles, air, common agents, and carriers. Among these, contact is the most important method, and implementing an appropriate disinfection policy can prevent this transmission route and significantly reduce the incidence of hospital infections (5).
In 2008, guidelines for disinfection and sterilization in health and medical centers were presented, emphasizing that any oversight or defects in the disinfection or sterilization of medical equipment pose a danger to the host and a threat for the transmission of infectious agents, such as viruses, from person to person. For instance, hepatitis B can involve the transmission of pathogenic agents from the environment to the patient. Therefore, effective disinfection and sterilization methods are essential to ensure that surfaces and medical and surgical instruments do not transmit infectious pathogens to patients (6).
Sodium hypochlorite (NaOCl) is a widely used disinfectant solution in healthcare centers, with varying ratios depending on its specific use. To disinfect surfaces using sodium hypochlorite, organic matter must be carefully cleaned to ensure high-efficiency disinfection. Due to the volatility of chlorine, a fresh solution of sodium hypochlorite should be used for each disinfection session (7).
Sayasept is another disinfectant that is widely used today, known for its very fast and effective action. This disinfectant is capable of destroying a wide range of microbes and can be used on medical and dental tools, equipment, and various surfaces. One of its advantages is that it is ready-to-use, alcohol-based, and compatible with a wide range of tools without causing corrosion (8-11).
The rate of nosocomial infections in a healthcare center reflects the quality of services provided, making it crucial to identify and control the causes of these infections. According to researchers, the best way to understand the prevalence of nosocomial infections is through short-term and limited studies (12-14). These features underscore the importance of correctly applying disinfection techniques in hospitals, particularly in specialized departments. Moreover, Kowsar Hospital has not previously conducted research of this nature.
2. Objectives
This study aimed to fill the research gaps by assessing the efficacy of sodium hypochlorite and Sayasept for disinfecting bacteria that cause nosocomial infections in the intensive care units of Kowsar Hospital. Additionally, there are few studies comparing the disinfection potential of Sayasept and sodium hypochlorite in preventing nosocomial infections in Iranian hospitals.
3. Methods
This study was conducted using a cross-sectional, descriptive-analytical methodology. To reduce the costs of the tests, four sampling points were selected (two pieces of equipment and two surfaces). Based on the obtained results, among the examined types of equipment, suction devices and ventilators were chosen for sampling (noting that suction samples will be taken from the inner part of the tracheal tube). Among the surfaces, the patient's breakfast table and the bar next to the patient's bed were selected based on conditions such as patient mobility and the presence or absence of a companion.
Samples were taken over three months from designated surfaces and equipment in several wards of Kowsar Hospital (male surgical, internal ICU, and post-CCU) following the National Infection Control Care System protocol. This was done without prior notice or sensitization of the staff to avoid any changes in behavior or errors. It should be noted that in addition to the service personnel carrying out the disinfection process, they were also responsible for implementing the plan by selecting comparable surfaces and equipment. This was done once by performing the preparation of disinfectant materials, the disinfection process, and sampling, and another time by performing only the disinfection process.
The steps for sampling were as follows:
(1) Initial coordination with the research vice-chancellor of the university and the hospital management to obtain permits for sampling surfaces and equipment in the special care departments of Kawsar Hospital, Semnan City.
(2) Sampling of surfaces (patient’s breakfast table, security bed rail) and equipment (ventilator, suction, and tracheal tube) in the special care departments of Kawsar Hospital.
(3) Performing the relevant tests in the reference health laboratory of Semnan health center.
The disinfection process was conducted three times per day in the hospital: During the morning shift (5:30 to 6:30 AM), the evening shift (12:30 to 1:30 PM), and at night (5:30 to 6:30 PM). Accordingly, the sampling procedures were conducted during all three shifts.
The first sampling run took place during the Saturday morning shift. Samples were collected before the disinfection process (at 5 AM) and then at one and a half hours (7 AM), two hours (9 AM), and four hours (11 AM) after disinfection.
The second sampling run was performed during the Sunday evening shift, with samples collected before disinfection (12 PM) and then at half an hour (1 PM), two hours (2 PM), and four hours (4 PM) after disinfection.
The third round of sampling occurred during the Monday night shift. Samples were collected one hour before disinfection (5 PM) and at half an hour (6:30 PM), two hours (8 PM), and four hours (11 PM) after disinfection.
Each sample was obtained over a three-month period, with the process conducted regularly across all shifts.
Place a 10 cm-diameter hollow circle on the Talc sheets before swabbing these Talcs sterilely in the environment and taking samples from the surfaces and equipment following the sampling instructions. After swabbing the nutrients from the desired locations, the swab was placed in sterile containers containing 1.5 cc of nutrient media and spun for 20 seconds. After two minutes of vortexing, the samples were incubated for one day at 35°C. Following the samples' incubation in a nutrient broth culture medium, the presence or absence of turbidity in the medium solution was used to determine microbial growth. The grown samples were then transferred to McConkey Agar (MAC) medium and incubated overnight at 35°C (15). After the necessary investigations, environmental isolates were identified using biochemical tests.
The catalase test was performed by transferring a wooden applicator from the center of a colony to a glass slide, immediately adding a drop of hydrogen peroxide to the colony on the slide, and observing the formation of bubbles. For the examined samples, differential tests for gram-negative and gram-positive organisms were conducted based on the bacteria's gram stain results to confirm the isolated isolates. If the organism was gram-negative, the following protocol was used: Several bacterial colonies were cultured on TSI Agar medium, and the depth was inoculated by piercing the medium. After incubation at 37°C for 24 hours, the results were interpreted. All the study steps are illustrated in Figure 1.
It was demonstrated that the production of hydrogen sulfide resulted in the formation of black sediment at the bottom of the tube and the emission of gas with cracks in the environment. A very small amount of the target organism was inoculated onto the surface of the Simon citrate agar gradient. After incubating the bacteria at 37°C, the appearance of a blue color indicated a positive reaction. Escherichia coli reacts negatively in this environment, with no color change observed. To investigate motility in a semi-solid medium, a tube containing SIM medium was used. After deep cultivation in this medium and incubation at 37°C for 24 hours, bacterial motility was indicated by growth not only along the inoculation line but also in the surrounding environment, showing clear growth or turbidity.
For gram-positive bacteria, the following differential tests were used to confirm the isolates: Gram staining was performed first, and if the organism appeared as cluster gram-positive cocci under the microscope, catalase, coagulase, DNase, and mannitol tests were conducted. Based on these test results, Staphylococcus bacteria were isolated (15).
Data were analyzed using the Statistical Package for Social Sciences (SPSS) software (version 25). Normality was assessed using skewness, the Shapiro-Wilk test, and kurtosis statistics. Repeated measures analysis of variance (RM ANOVA) was conducted to compare baseline and follow-up. An analysis of covariance (ANCOVA) was used to identify between-group changes in the primary and secondary outcomes. A P-value < 0.05 was considered statistically significant.
All of the chemical reagents used in the present study were of analytical grade and did not require further purification.
4. Results
Disinfection with sodium hypochlorite in the surgical ICU resulted in negative culture results 4 hours after disinfection. This could be due to less movement of patients or companions in this department, reducing the likelihood of contamination during disinfection intervals. In contrast, samples from the patient's bedside table showed that disinfection with Sayasept produced negative culture results only half an hour after disinfection in both the CCU and ICU surgical areas.
For samples taken from the patient's breakfast table, disinfection with Sayasept resulted in negative culture results 4 hours after disinfection in both the CCU and ICU surgical areas. However, disinfection with Sayasept did not affect the microorganisms present in the ventilator and bi-pep. This could be because these devices are used regularly, reducing the opportunity for effective disinfection each time they are used. In the CCU, sodium hypochlorite disinfection resulted in a negative culture 4 hours after disinfection. Additionally, negative culture results were obtained in the surgical ICU just 30 minutes and 2 hours after disinfection with sodium hypochlorite (Table 1). The high volume of patient or companion movement in other departments might have decreased the likelihood of achieving negative culture results during disinfection intervals.
| Type of Microorganism | Time of Investigation | |||
|---|---|---|---|---|
| Before Disinfection | Half an Hour After Disinfection | 2 Hours After Disinfection | 4 Hours After Disinfection | |
| Staphylococcus | - | - | - | - |
| Klebsiella | - | 1 (20.0) | 1 (20.0) | 2 (66.7) |
| Enterobacter | 1 (16.7) | - | - | - |
| Citrobacter | 5 (83.3) | 4 (80.0) | 4 (80.0) | 1 (33.3) |
| Total | 6 (100) | 5 (100) | 5 (100) | 3 (100) |
Distribution of Microorganisms Grown in Different Departments of Kowsar Hospital, Semnan, Iran (2021) After Disinfection with Sodium Hypochlorite at Different Times a
In the CCU, disinfection with Sayasept on the patient's bedside table resulted in negative culture results only half an hour after disinfection. However, in the surgical and internal ICU for men, negative culture results were achieved 2 hours after disinfection. For samples taken from the patient's breakfast table in the CCU and the men's ward, negative culture results were observed only half an hour and 2 hours after disinfection. In contrast, in the surgical ICU, negative culture results were obtained only 4 hours after disinfection. Disinfection in bi-pep (inside men's wards) resulted in negative culture results 2 hours after disinfection, whereas disinfection with Sayasept did not affect the microorganisms in the ventilator. This may be due to the frequent use of ventilator devices, which were followed by disinfection with sodium hypochlorite.
According to the table, disinfection with sodium hypochlorite in the surgical ICU resulted in negative culture results 4 hours after disinfection. In contrast, disinfection with sodium hypochlorite in the CCU did not affect the microorganisms present in the suction. This discrepancy could be due to the higher rate of patient admissions and discharges in the CCU compared to the ICU, which may reduce the likelihood of achieving negative culture results during disinfection intervals.
For samples taken from the patient's bedside table, disinfection with Sayasept in the CCU resulted in negative culture results only 1.5 and 4 hours after disinfection. In the surgical ICU, however, Sayasept consistently produced negative culture results. In samples from the patient's breakfast table, the CCU and surgical ICU both showed negative culture results 4 hours after disinfection, while in the men's wards, negative results were achieved only half an hour after disinfection.
Disinfection with Sayasept did not affect the microorganisms present in the ventilator and bi-pep, likely due to the frequent use of these devices.
According to Table 1, Citrobacter was the most common microorganism grown at all investigated times. The results of the ANOVA analysis indicated a significant reduction in the number of microorganisms grown after disinfection with sodium hypochlorite (P-value = 0.042), demonstrating its high effectiveness.
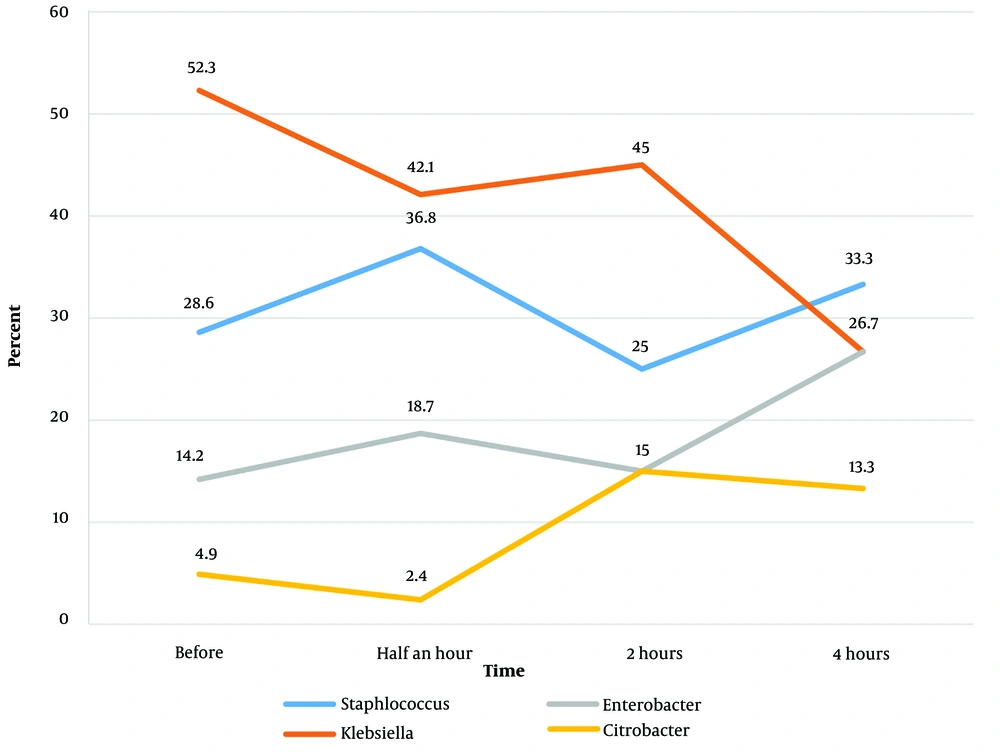
Table 2 presents the distribution of microorganisms grown at different times following disinfection with Sayasept. According to this table, Klebsiella was the most common microorganism grown at all investigated times except 4 hours after disinfection. Figure 2 shows the distribution of microorganisms grown at different times of disinfection.
| Type of Microorganism | Time of Investigation | |||
|---|---|---|---|---|
| Before Disinfection | Half an Hour After Disinfection | 2 Hours After Disinfection | 4 Hours After Disinfection | |
| Staphylococcus | 6 (28.6) | 7 (36.8) | 5 (25.0) | 5 (33.3) |
| Klebsiella | 11 (52.3) | 8 (42.1) | 9 (45.0) | 4 (26.7) |
| Enterobacter | 3 (14.2) | 3 (18.7) | 3 (15.0) | 4 (26.7) |
| Citrobacter | 1 (4.9) | 1 (2.4) | 3 (15.0) | 2 (13.3) |
| Total | 21 (100) | 19 (100) | 20 (100) | 15 (100) |
The distribution of the Type of Microorganisms Grown in the Different Investigated Departments of Kowsar Hospital, Semnan, Iran (2021) after Disinfection with Sayasept at Different Times a
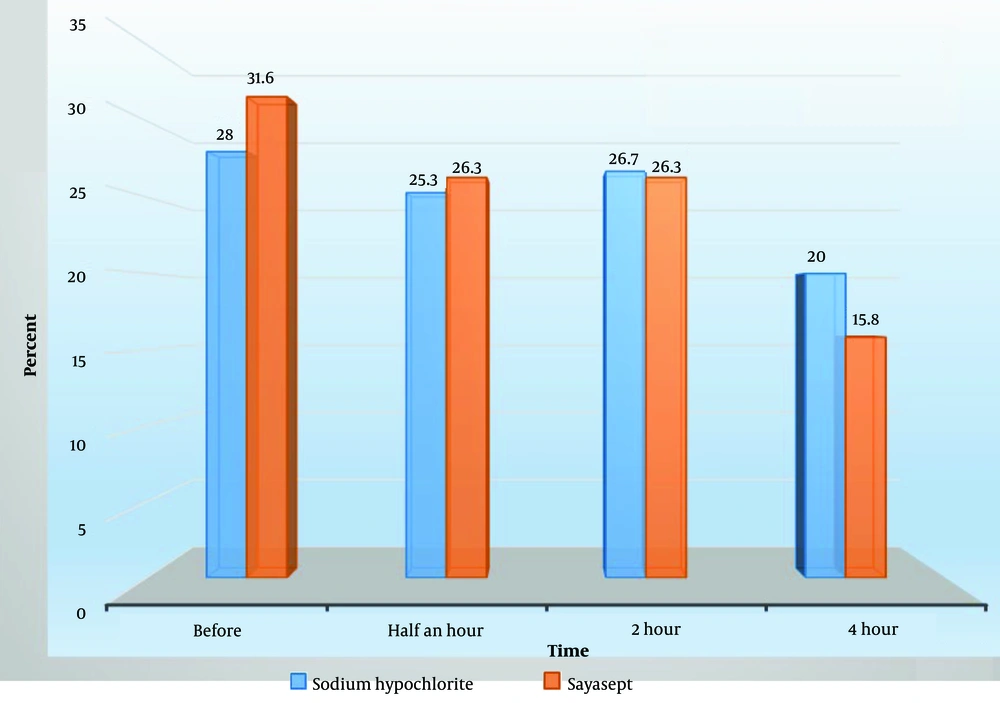
According to Table 3, the highest number of microorganisms grown after disinfection with sodium hypochlorite occurred half an hour and 2 hours after disinfection (26.3%). Similarly, the highest number of microorganisms grown after disinfection with Sayasept was observed 2 hours after disinfection (26.7%).
| Time of Investigation | Number of Positive Cultures | |
|---|---|---|
| Sodium Hypochlorite | Sayasept | |
| Before disinfection | 21 (28.0) | 6 (31.6) |
| Half an hour after disinfection | 19 (25.3) | 5 (26.3) |
| 2 hours after disinfection | 20 (26.7) | 5 (26.3) |
| 4 hours after disinfection | 15 (20.0) | 3 (15.8) |
| Total | 75 (100) | 19 (100) |
Distribution of Positive Findings Regarding the Microorganisms Grown in Different Departments of Kowsar Hospital, Semnan, Iran (2021) According to the Type of Disinfectant Used at Various Times a
Figure 3 shows the distribution of microorganisms grown at different times of disinfection. The results of the ANOVA analysis indicated that disinfection with sodium hypochlorite caused a significant decrease in the number of microorganisms grown after disinfection compared to before (P-value = 0.042). However, disinfection with Sayasept did not have a statistically significant effect on the number of microorganisms grown after disinfection compared to before (P-value = 0.132). This demonstrates the high effectiveness of disinfection with sodium hypochlorite.
5. Discussion
Based on the findings, 81.8% (or 27 instances) of the 33 samples—11 samples taken over three work shifts—cultured various pathogens before the disinfection procedure. The World Health Organization reported in 2011 that the prevalence of microbiological agents in hospital treatment levels increased globally from 5.7% to 19.1%. It also noted that the prevalence of microbiological agents in hospital settings is much higher in low-income countries (15.5%). Additionally, in high-income and European nations, the net prevalence of microbiological agents at the hospital level is 1.7% and 7.6%, respectively (16), which are lower than the results obtained in the present study.
In internal studies conducted in Iranian hospitals, researchers concluded that the prevalence of microbial agents at hospital levels was more than 25% (17). According to these statistics, the intensive and internal care departments of the Men's Hospital in Semnan show higher prevalence rates. The subjects investigated in the current study did not have a favorable status among the hospitals under internal investigation. The reason for these discrepancies and differences in the results of various studies could be related to the sampling process in the present study. The sampling procedure was conducted before the disinfection process, thereby increasing the possibility of microorganism growth.
The frequent contact of patients and medical staff with the surfaces examined in this study is not unexpected. Zazouli et al. in Mazandaran also reported that the amount of microbial contamination before the disinfection process in the ear, nose, and throat department of the investigated hospital was 80% (18), which is close to the results of the present study. Additionally, other studies have indicated that the prevalence of hospital infections varies across different medical centers and depends on numerous factors, including medical interventions, hospital conditions, and individual characteristics (19).
To address this issue, the health departments of Semnan University of Medical Sciences are properly monitored. Measures include training and equipping the armed forces, encouraging medical staff to adhere to health principles, publishing and distributing health notices, placing health placards in prominent locations, providing training to hospital staff, issuing warnings when necessary—particularly to patients' companions—and effectively managing patient flow to treatment departments such as ICU and CCU (20). These actions can improve health conditions and reduce the spread of microbial agents at Kowsar Hospital.
In this study, we investigated the effectiveness of the disinfection process on bacteria causing hospital infections in the intensive care units. One significant factor in the spread of hospital infections is the improper use of disinfectants (3). Using effective and safe disinfectant solutions that cause minimal damage to equipment and personnel is a fundamental principle of disinfection. The results of the present study indicated that disinfection with sodium hypochlorite caused a significant decrease in the number of microorganisms grown after disinfection compared to before; however, disinfection with Sayasept did not have a significant effect on the number of microorganisms grown after disinfection compared to before.
Because there are limited studies comparing these two disinfectants, discussion about their effects on hospital infections is rare. In a study by Yousefi et al. in Hamedan, the effectiveness of common disinfectants on strains isolated from teaching hospitals in Hamedan was investigated.
This study was a laboratory experiment that examined a total of 400 samples from the ICU, CCU, burn, and operating room departments of teaching hospitals. The results demonstrated that sodium hypochlorite was significantly effective against Staphylococcus and E. coli strains, aligning with the findings of the present study (21). One reason for the significant effect of disinfection with sodium hypochlorite on the reduction of microorganisms post-disinfection compared to pre-disinfection could be attributed to its common use at Kowsar Semnan hospital. The experience and frequent use of this disinfectant likely led to proper formulation and application by the service personnel. The appropriate application of this disinfectant is crucial, as other studies have also stated that for effective disinfection using sodium hypochlorite, organic materials must be carefully cleaned to ensure high efficiency. Due to the volatility of chlorine, a fresh solution of sodium hypochlorite should be used each time for disinfection (7).
Other studies have declared that the efficacy of sodium hypochlorite in killing pathogens depends on the percentage of active chlorine in the solution, as well as the solution's pH and temperature. Increasing the percentage of active chlorine, reducing the pH, and increasing the temperature can enhance its disinfection effect (22-24).
In a cross-sectional study conducted in the different wards of Allameh Behlul Hospital in Gonabad, Iran, a total of 245 samples were studied before and after disinfection with four different disinfectants: Deconnex AF50, Peranacide M1, Microzed GPH, and Sarphosepte Quicks. The samples were cultured, investigated by biochemical tests, and the number of colonies was determined. The results were analyzed using the Wilcoxon statistical test, which reported that the most common gram-positive and gram-negative bacteria were Staphylococcus epidermidis and E. coli, respectively. Moreover, Deconnex and Microzed GPH disinfectants were significantly effective in all parts (P < 0.05), whereas disinfection with Peranacide was not statistically significant (P > 0.05) (25).
In a similar study, Singh et al. evaluated seven disinfectants at the concentrations recommended by their manufacturers on both rough and smooth surfaces in an Indian hospital setting. These surfaces were experimentally contaminated with methicillin-resistant Staphylococcus aureus, Acinetobacter baumannii, Klebsiella pneumoniae, Enterobacter aerogenes, Pseudomonas aeruginosa strains, a standard isolate of Salmonella typhi, and Candida albicans. The highest average log reduction of tested microbes on rough and smooth surfaces were achieved by DesNet (5.05) and Lysol (5.68), respectively (26).
As with any study, there were limitations. One limitation was the presence of numerous known and unknown factors that may have influenced the results. However, efforts were made to control as many of these variables as possible to ensure accurate results and broader generalizability. Another limitation was that the study was conducted at a single center, which inevitably introduced selection bias. Additionally, due to the constraints of the measurement tools, the study could not examine other microorganisms, particularly fungi, on therapeutic surfaces. Future studies should address this gap. Nonetheless, this study aims to compare two common disinfection methods, providing valuable insights for senior managers and health engineers to better prevent nosocomial infections.
5.1. Conclusions
Overall, the study's findings showed that there were significantly more microorganisms grown in the sections under investigation prior to disinfection compared to after using sodium hypochlorite for disinfection. In contrast, there was a significant drop in the number of microorganisms grown in the sections following disinfection compared to before. However, Sayasept disinfection had no appreciable impact on the quantity of microorganisms that developed after disinfection compared to before. Additionally, it is advised that health policies be established to identify appropriate disinfectants for use in hospitals and to provide adequate training facilities, particularly for the use of sodium hypochlorite. It is suggested that future studies focus on addressing the mentioned limitations and identifying unknown factors that may have influenced the present results. Moreover, utilizing artificial intelligence can be a useful tool to improve the disinfection process, diagnose errors, and understand their causes.