1. Background
Subclinical hypothyroidism (SCH) is identified by serum thyroid-stimulating hormone (TSH) levels above 5 mIU/L and normal thyroxine levels (fT4) (1). Subclinical hypothyroidism has a prevalence of 12% to 18% in the general population (2, 3) but is found in only about 2% of children and adolescents (4). Subclinical hypothyroidism may evolve into overt hypothyroidism (OH) at a rate of approximately 2% per year, potentially leading to significant health issues (5-7). While thyroid hormone replacement with levothyroxine is the standard treatment for hypothyroidism (8), the management of SCH remains a subject of debate (9). Treatment for SCH typically depends on the presence of clinical symptoms indicative of hypothyroidism, the extent of TSH elevation, and changes in TSH and T4 levels over time. Treatment is generally recommended for TSH levels above 10 mIU/L according to most guidelines (2), and may also be considered for patients with TSH levels between 5 - 10 mIU/L accompanied by symptoms of hypothyroidism (10). Therefore, continuous monitoring is essential in children with SCH to evaluate potential risks associated with increased serum TSH levels.
Research indicates that SCH is linked to insulin resistance (IR), dyslipidemia, diastolic dysfunction, coronary disease, and heart failure in adults (11-14). Insulin resistance significantly contributes to cardiovascular disease development and atherosclerosis (15, 16). Limited studies suggest that children and adolescents with SCH might also display adverse cardiovascular risk profiles (17-20).
The treatment of SCH in children and adolescents is controversial. Nonetheless, addressing metabolic impairments that result from SCH is a crucial aspect of managing this condition.
2. Objectives
This study aimed to explore the relationship between IR and hypothyroidism in children and adolescents aged 4 to 18 years.
3. Methods
3.1. Participants
This case-control study included children and adolescents aged 4 to 18 years. The study involved 60 participants divided equally into case (n = 30) and control (n = 30) groups. The case group comprised 30 patients referred to the Endocrinology Clinic of Imam Reza, Shiraz, Iran, due to abnormal thyroid function tests and selected using a convenience sampling method. A case was defined as a child or adolescent aged 4 to 18 diagnosed with SCH (normal T4 and TSH > 5). The control group consisted of 30 healthy children and adolescents with normal thyroid tests, randomly selected during routine check-ups. Both groups were matched in terms of gender, age, weight, height, and Body Mass Index (BMI). Exclusion criteria included positive Anti-TPO antibodies, goiter, a history of thyroid disease, systemic diseases, chronic or acute diseases, diabetes, and a BMI ≥ 85% percentile for age and sex to avoid confounding effects of obesity and overweight, which can induce IR and metabolic syndrome.
The sample size was initially calculated to be 15 samples per group based on the study by Enzevaei et al. (21), using a significance level (α) of 0.05 and a power (β) of 0.95. However, to account for potential drop-outs, the number was increased to 30 participants per group.
3.2. Technical Information
All subjects underwent a thyroid examination by a Pediatric Endocrinologist, and TSH and T4 levels were re-evaluated. Additionally, anti-thyroid peroxidase levels were measured in the case group. TSH levels were determined using an Electro-chemiluminescent kit from Roche Life Science, Germany. Fasting insulin and fasting blood sugar (FBS), along with lipid profiles including triglycerides (TG), cholesterol (Chol), low-density lipoprotein (LDL), and high-density lipoproteins (HDL), were all measured after at least 12 hours of fasting. Insulin was also quantified using an Electro-chemiluminescent kit from Roche Life Science, Germany. Insulin resistance was assessed using the homeostatic model assessment for insulin resistance (HOMA-IR), calculated with the formula: Glucose (mmol/L) × insulin (mIU/L)/22.5. The threshold for HOMA-IR indicating IR in non-diabetic children is set at > 1.775 (22, 23). Odds ratios (ORs) and 95% confidence intervals (CIs) were subsequently calculated.
3.3. Statistical Analysis
Data analysis was conducted using SPSS software, version 25.0 (SPSS Inc., Chicago, IL, USA). The t-test was employed to compare the means of quantitative data, and Pearson's correlation coefficient was used to assess statistical associations between insulin, HOMA-IR, and other variables. The chi-square test was utilized for analyzing associations among qualitative variables. A P-value of less than 0.05 was considered statistically significant.
4. Results
Thirty children and adolescents were included in each group. The mean age was 8.99 ± 2.35 for the case group and 8.91 ± 2.34 for the control group. All participants maintained a normal BMI with weight percentiles ranging from 5 to 85.
No significant differences were found in the average age (P = 0.900), height (P = 0.792), weight (P = 0.513), or BMI (P = 0.193) between the case and control groups (Table 1).
| Variables | Case Group (n = 30) | Control Group (n = 30) | P-Value |
|---|---|---|---|
| Gender | |||
| Male | 14 (46.7) | 13 (43.3) | 0.519 b |
| Female | 16 (53.3) | 17 (56.7) | |
| Age, y | 8.993 ± 2.358 | 8.917 ± 2.344 | 0.900 c |
| Height, cm | 130.387 ± 13.296 | 131.327 ± 14.228 | 0.792 c |
| Weight, kg | 28.185 ± 7.889 | 29.643 ± 9.236 | 0.513 c |
| Body Mass Index, kg/m2 | 16.193 ± 1.523 | 16.857 ± 1.776 | 0.193 c |
a Values are expressed as mean ± SD or No. (%).
b Based on the Chi-square test.
c Based on the independent t-test.
t-tests revealed no significant differences in the mean levels of HDL (P = 0.724), LDL (P = 0.573), cholesterol (P = 0.324), TG (P = 0.788), and T4 (P = 0.332) between the two groups. However, significant differences were observed in TSH levels (P < 0.001), serum insulin levels (P = 0.001), HOMA-IR (P = 0.001), and FBS (P = 0.042) in the case group compared to the control group (Table 2).
| Variables | Case Group (n = 30) | Control Group (n = 30) | P-Value b |
|---|---|---|---|
| Lipid profile | |||
| TG, mg/dl | 92.30 ± 31.865 | 94.37 ± 27.126 | 0.788 |
| Chol, mg/dl | 155.70 ± 21.966 | 150.23 ± 20.604 | 0.324 |
| LDL, mg/dl | 79.73 ± 16.830 | 77.53 ± 12.945 | 0.573 |
| HDL, mg/dl | 51.13 ± 11.020 | 50.23 ± 8.427 | 0.724 |
| Thyroid function test | |||
| TSH, mU/L | 8.13 ± 1.299 | 2.67 ± 1.036 | < 0.001 |
| T4, mcg/dl | 8.38 ± 1.183 | 8.72 ± 1.439 | 0.322 |
| FBS, mg/dl | 93.77 ± 7.272 | 90.30 ± 5.535 | 0.042 |
| Insulin, µlU/ml | 8.40 ± 4.224 | 5.38 ± 1.611 | 0.001 |
| HOMA-IR | 1.97 ± 1.089 | 1.18 ± 0.371 | 0.001 |
Abbreviations: TG, triglyceride; Chol, cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TFT, thyroid function test; TSH, thyroid-stimulating hormone; T4, thyroxine; FBS, fasting blood sugar; HOMA-IR, homeostatic model assessment for insulin resistance.
a Values are expressed as mean ± SD.
b Based on the independent t-test.
There was a significant correlation between insulin levels and fasting blood sugar (FBS) (P = 0.018), and insulin levels and TSH (P < 0.001), as well as insulin levels and LDL (P = 0.029) in the case group. Additionally, significant relationships were observed between the HOMA-IR and FBS (P = 0.002), TSH (P < 0.001), and LDL (P=0.025) (Table 3).
| Variables | Insulin Levels | HOMA-IR | ||
|---|---|---|---|---|
| r | P-Value | r | P-Value | |
| Age, y | 0.440 a | 0.015 | 0.439 a | 0.015 |
| Height, cm | 0.531 b | 0.003 | 0.523 b | 0.003 |
| Weight, kg | 0.507 b | 0.004 | 0.502 b | 0.005 |
| BMI, kg/m2 | 0.422 a | 0.020 | 0.428 a | 0.018 |
| TG, mg/dl | 0.096 | 0.613 | 0.100 | 0.599 |
| Chol, mg/dl | 0.188 | 0.320 | 0.203 | 0.282 |
| LDL, mg/dl | 0.398 a | 0.029 | 0.408 a | 0.025 |
| HDL, mg/dl | -0.028 | 0.882 | -0.014 | 0.943 |
| TSH, mU /dl | 0.661 b | < 0.001 | 0.641 b | < 0.001 |
| T4, mcg/dl | -0.097 | 0.611 | -0.088 | 0.646 |
| FBS, mg/dl | 0.430 a | 0.018 | 0.545 b | 0.002 |
Abbreviations: SCH, subclinical hypothyroidism, BMI, Body Mass Index; TG, triglyceride; Chol, cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TSH, thyroid-stimulating hormone; T4, thyroxine; FBS, Fasting blood sugar; HOMA-IR, homeostatic model assessment for insulin resistance.
a Correlation is significant at the 0.05 level (2-tailed).
b Correlation is significant at the 0.01 level (2-tailed).
Analysis of lipid parameters, including high-density lipoprotein (HDL), LDL, cholesterol, and triglycerides, revealed that triglyceride levels exceeded the normal range (< 150 mg/dl) in two cases from the case group and one child from the control group. Cholesterol levels were above the normal range (< 170 mg/dl) in six individuals (20%) from the case group and four individuals (13.33%) from the control group. LDL levels were above the normal range (< 100 mg/dl) for two subjects in the control group (102 and 116 mg/dl) and three patients in the case group (122, 112, and 109 mg/dl), although levels between 100 and 129 mg/dl are considered acceptable for individuals without health issues.
In the control group, all subjects had TSH levels within the normal range (< 4.30 mU/L). In contrast, TSH levels in the case group ranged from 6 mU/L to 10.70 mU/L. Thyroxine (T4) levels were within the normal range (4.5 - 11.2 mcg/dl) for all subjects.
The average fasting blood sugar was 92.03 ± 6.641 mg/dl (range: 79 - 113 mg/dl). FBS levels slightly exceeded the normal range (< 100 mg/dl) in seven subjects (23.33%) from the case group and two subjects (6.67%) from the control group.
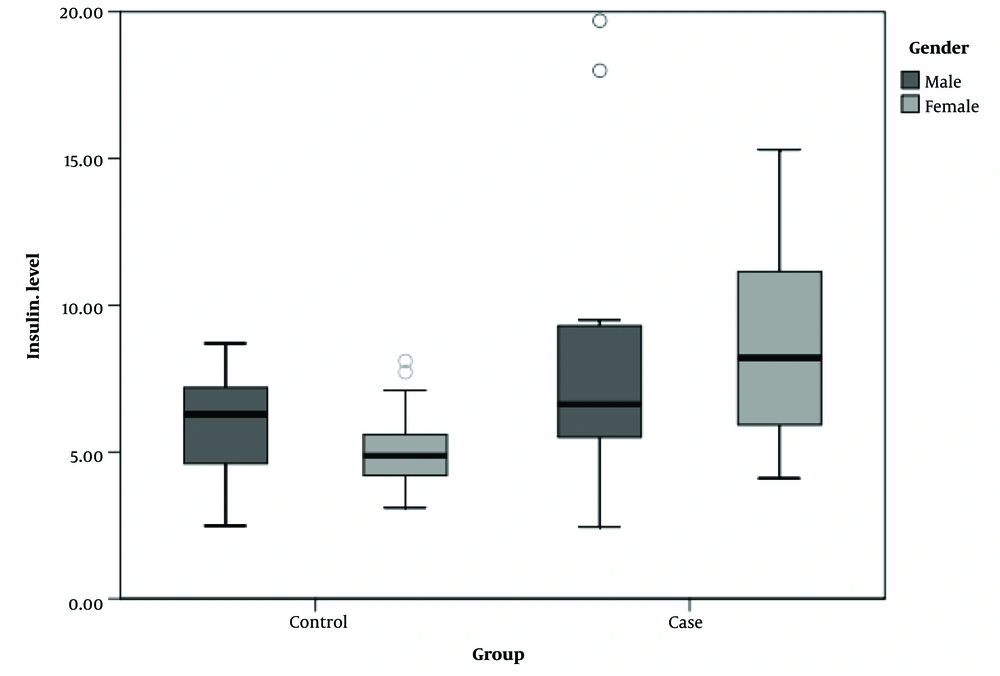
As depicted in Figure 1, serum insulin levels ranged from 2.44 µIU/ml to 19.70 µIU/ml in the case group and from 2.48 µIU/ml to 8.70 µIU/ml in the control groups, segmented by gender.
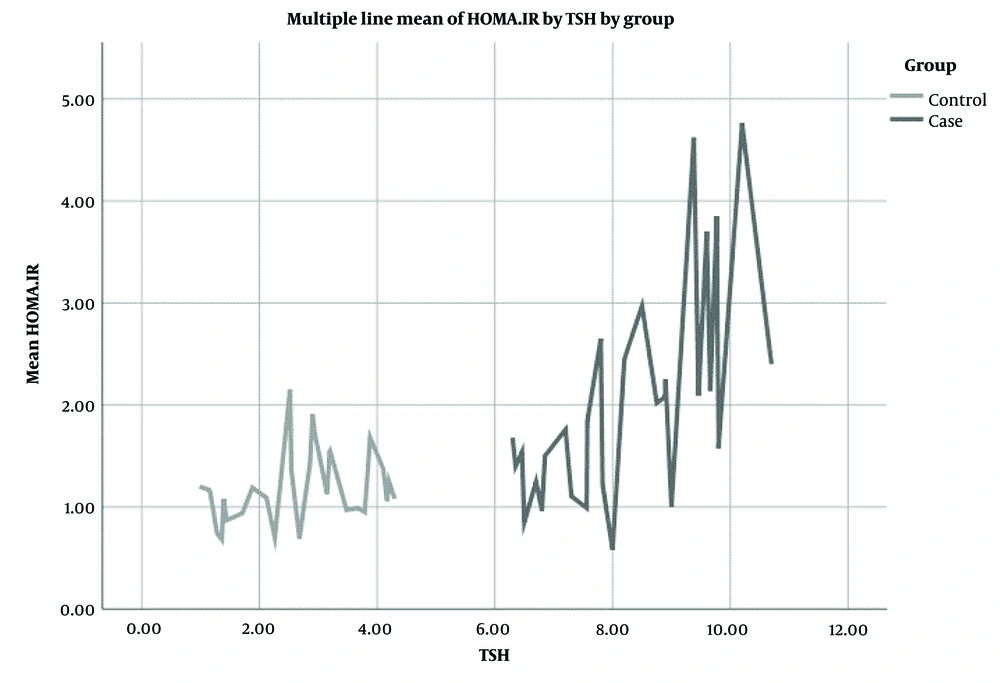
As illustrated in Figure 2, the mean HOMA-IR significantly correlated with TSH levels in the case group compared to the control group.
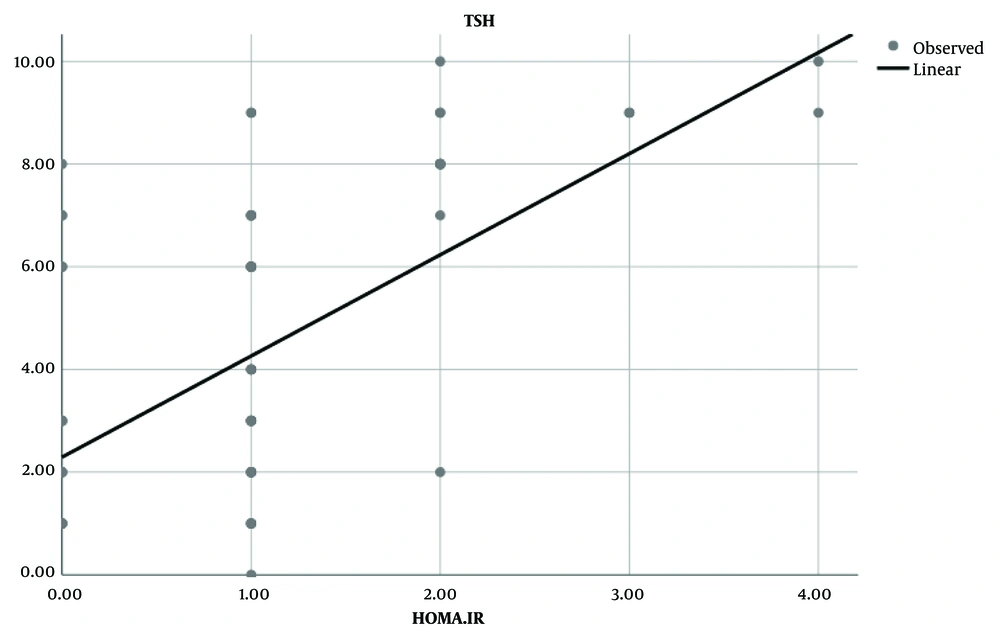
In the case group, TSH levels demonstrated a positive and linear correlation with HOMA-IR, unlike in the control group (Figure 3). According to the HOMA-IR threshold for non-diabetic children (> 1.775), no cases of IR were found in the control group, whereas four patients (13.33%) exhibited IR in the case group and two subjects (6.66%) had HOMA-IR levels exceeding 2.6.
5. Discussion
Subclinical hypothyroidism is the predominant thyroid disorder among children and adolescents (24). Recent research has established a significant association between SCH and IR (5, 25), though this link is not universally acknowledged (26). Our study reveals a direct correlation between TSH levels and insulin levels, with insulin levels markedly rising as TSH levels increase in the patient group. Additionally, both insulin levels and the HOMA-IR were notably higher in SCH patients compared to their euthyroid counterparts. This supports the notion that elevated TSH levels enhance the risk of IR. Similarly, Vyakaranam et al. found that both mean insulin levels and HOMA-IR were significantly higher in individuals with SCH compared to euthyroid individuals, and that TSH levels were positively correlated with insulin levels and HOMA-IR (27).
Maratou et al. also noted an increase in HOMA-IR among patients with SCH compared to euthyroid subjects, alongside reduced glucose transport rates (11). Roos et al. confirmed a significant association between TSH and HOMA-IR in SCH patients (28). Conversely, Al Sayed et al. reported no significant differences in HOMA-IR between SCH patients and the control group (29). These findings, aligned with ours, underscore the significant relationship between SCH and IR.
Yadav et al. explored cardiovascular risk factors in children with SCH, discovering that BMI, waist circumference, waist-to-height ratio, LDL cholesterol, TG, fasting insulin, and HOMA-IR were all significantly elevated in SCH subjects compared to euthyroid controls (17).
Insulin resistance is known to contribute to metabolic syndrome and cardiovascular complications in SCH patients (27). Additionally, IR plays a crucial role in dyslipidemia, an important factor in the development of atherosclerosis and cardiovascular disease in both children and adults. Duntas and Wartofsky highlighted how SCH can disrupt cholesterol and lipoprotein metabolism, thereby increasing the risk of atherosclerosis and cardiovascular diseases (12). Furthermore, a meta-analysis including eight observational studies indicated that SCH is linked with increased carotid intima-media thickness, possibly due to elevated TSH levels (13).
An important consideration is when to treat SCH in children. It is established that thyroid hormone replacement should be recommended for children with autoimmune thyroiditis and a progressive increase in TSH levels over time, especially if there are symptoms of hypothyroidism, the presence of a goiter, or other autoimmune diseases such as diabetes. Levothyroxine may also be advised for children and adolescents with SCH who exhibit proatherogenic metabolic abnormalities. Conversely, children with mild SCH who do not exhibit goiter or hypothyroid symptoms and who test negative for anti-thyroid autoantibodies may only require monitoring (19). In this study, non-obese children and adolescents with SCH who neither had a goiter nor tested positive for Anti-Tpo were examined. The findings indicate that non-obese children with SCH may develop IR. Given that IR is a significant risk factor for future cardiovascular disease, these children and adolescents should be monitored for metabolic complications.
In this study, the risk of cardiovascular disease was assessed using the HOMA-IR cut-off threshold (≥ 2.6) for cardiovascular disease in pediatrics (30). The results demonstrated that SCH increased the risk of cardiovascular disease by 16.18 times in subjects with SCH compared to the control group.
Given that the treatment of SCH in children remains controversial and IR can lead to an increased risk of cardiovascular diseases, it is recommended to closely monitor these potential adverse metabolic effects of SCH.
A larger sample size could have yielded more precise results. Consequently, future research with more extensive participant groups is warranted to more accurately investigate IR in children with SCH. Additionally, long-term follow-up studies are recommended to determine the clinical relevance of these findings in children and adolescents with SCH.
5.1. Strengths and Limitations
Currently, there is no consensus on treating SCH in children, and its treatment often does not account for the metabolic implications of the condition. Given that IR heightens the risk of cardiovascular disorders—one of the most significant health issues in adulthood—the insights from this study regarding the link between TSH and IR levels in children with SCH could expedite the early initiation of treatment for SCH, thereby helping to avert secondary complications. However, this study's limitation is its small sample size available at the time of research. As the prevalence of the disease during data collection can influence the findings, caution should be exercised when generalizing these results. Conducting this study across different ethnic groups and ages with a larger population might yield more comprehensive insights.
5.2. Conclusions
Our findings demonstrate a positive correlation between TSH levels and IR in children with SCH. Furthermore, HOMA-IR significantly increased with TSH elevation in children with SCH compared to their healthy counterparts. Thus, assessing IR and initiating timely treatment in patients with SCH to prevent future complications is advisable.