1. Context
Conflict of interest has always been one of the challenges of healthcare systems (1, 2). Decisions on prioritizing, assigning, and distributing resources are among the most important measures taken by healthcare systems (3). However, like other sectors, the decision-making process in healthcare is influenced by non-technical and non-price factors, such as conflicts of interest (4). The Organization for Economic Cooperation and Development’s (OECD) guidelines define conflict of interest as a conflict between governmental tasks and government agents' interests, where these interests can wrongfully affect their duties (5). Therefore, conflict of interest refers to situations that lead professional decisions and measures to be swayed by secondary personal interests (6).
The development of new technologies in medical industries, including pharmaceuticals and medical equipment, has exposed the medical community to new challenges. These challenges stem from the conflict between medicine as a profession and the industry or business aiming for profit (7). Conflict of interest affects the quality of patient treatment, the consistency of research, the focus of education, and the trust of society (8). According to the OECD, high-risk areas for conflicts of interest include secondary employment, information rents, gifts, and other forms of benefits, as well as personal, family, and social opportunities; simultaneous external positions and activities in private organizations after leaving a job (9). This conflict can potentially have an inappropriate effect on the best practice and clinical decisions of physicians regarding patients, leading to physical, mental, and financial outcomes (7).
The pharmaceutical sector is one of the most important components of healthcare systems (10). The pharmaceutical market's high value, complicated drug supply chain processes, and the intricate assignment of players with different duties make the drug industry more prone to corruption (11). For a long time, financial relationships between health professionals and drug producers have been the subject of international discussions. Numerous factors can hinder the implementation of ethical systems in pharmaceutical issues. For example, global pharmaceutical companies with high competitiveness and advertising abilities can affect not only ethics but also the pharmaceutical and educational systems through financial incentives (12). Pharmaceutical companies invest considerable funds in interacting with health professionals. In 2013, 20 pharmaceutical companies spent 14$ billion on journal advertising, traditional detailing, professional meetings, and e-promotion (13). In the pharmaceutical system, reviews of various studies indicate the existence of conflicts of interest in different fields, including regulatory, policymaking, and governance systems such as the United States Food and Drug Administration, the Irish Medicine Board, the British Medicines Agency, and the European Union Medicine Agency (9). These relationships can lead to conflicts of interest, negatively affecting patient care and increasing healthcare costs (14, 15). Most specialists or experienced healthcare providers are potentially subject to conflicts of interest. Thus, some consider managing conflicts of interest before resolving them.
Numerous methods have been proposed to manage conflicts of interest, such as restricting interactions and problematic activities, making conflicts of interest transparent to manage them, training healthcare providers to detect and deal with conflict of interest situations, and industry self-regulation (16). However, despite concerns about conflicts of interest, their management in medicine is not well documented, and policies in this sector are executed inconsistently. For example, in a study of 95 medical organizations publishing guidelines, it was found that 63% of organizations had accepted industry budgets, while only 1% of guidelines had publicized this issue (17). Even in studies where publicizing conflicts of interest is typical, inconsistencies exist in providing information, and about one-third of researchers have a conflict of interest (18).
2. Objectives
The investigation of the studies showed that there are no reviews regarding strategies for managing conflicts of interest in the pharmaceutical industry. Therefore, the current study aimed to review strategies to manage conflicts of interest in pharmaceutical sectors.
3. Methods
This systematic review was conducted in accordance with the PRISMA guideline (19) in 2024.
3.1. Eligibility Criteria
In this review, medical sciences resources such as EMBASE and PubMed were searched due to their relevant documents on medical sciences, especially pharmaceutical studies. Citation databases, including Scopus and Web of Science, were also searched to access more related documents. These resources covered English-language documents published before 2000. Google Scholar was also searched for gray literature, which may be indexed in different databases.
3.2. Information Sources
To achieve the aim of the study, which was a review of the strategies to manage conflicts of interest in pharmaceutical sectors, EMBASE, PubMed, Web of Science, Scopus, and Google Scholar were searched without a time limitation in March 2024. The search keywords were "conflict of interest" and "pharmaceutical industry," along with their synonyms in Medical Subject Headings (MeSH) and EMTREE. The databases were searched based on the PICOT formula in PubMed:
("interest conflict"[tiab] OR "conflicting interests"[tiab] OR "conflict* of interest*"[tiab] OR "conflict-of-interest"[tiab] OR "clash* of interests"[tiab] OR "contradictory interests"[tiab] OR "opposing interests"[tiab] OR "competing interests"[tiab] OR "conflicting objectives"[tiab] OR "divergent interests"[tiab]) AND ((industry[tiab] AND pharmaceutic*[tiab]) OR "pharmaceutical industry"[tiab] OR (industry[tiab] AND drug[tiab]) OR "drug industries"[tiab] OR "pharmaceutical system"[tiab])
- Phenomenon of Interest: Articles dealing with the concept of conflict of interest and strategies to manage it.
- Context: Articles focusing on pharmaceutical activities.
- Outcome: Articles proposing a possible strategy to manage conflicts of interest in the pharmaceutical sector.
- Time: Without time limitation.
The search strategy was adapted based on the characteristics of each database. The search was conducted by a medical library and information science specialist.
3.3. Selection of Sources of Evidence
The references of related articles were also screened to find more relevant articles. Inclusion criteria were original and review articles and gray literature related to management strategies for conflicts of interest in pharmaceutical sectors, English articles, and having full texts, without time limitation until 2024. Letters, letters to the editor, case studies, editorials, systematic reviews, comments, opinions, perspectives, books, and conference papers were excluded from the review. The abstracts of retrieved records were entered into EndNote x8. After removing duplicates, the titles and abstracts of records were screened, and related documents were identified. This phase was repeated by two reviewers independently, and disputed cases were resolved by consulting a third person. Finally, the full text of the related studies was reviewed by two reviewers independently, and disagreements were resolved by consulting with a third person. The bibliographic characteristics of each record, including the first author, the year of the study, the place of the study, the aim of the study, the sample, data collection tools, and strategies regarding the management of conflicts of interest, were recorded in a data extraction form designed in MS Word 2016.
3.4. Critical Appraisal of Individual Sources of Evidence
The Joanna Briggs Institute (JBI) checklists were used for the critical appraisal of cross-sectional studies (8 items) (20) and qualitative studies (10 items) (21). Studies with a score above 85%, between 85% and 75%, between 75% and 55%, and below 55% were categorized as "excellent quality," "very good," "good," and "poor quality," respectively. The qualification of the evidence was conducted independently by two reviewers. In the case of disagreement, the article was assessed by a third person.
3.5. Synthesis of Results
Braun and Clark's model was used for thematic content analysis (22). The data analysis process involved becoming familiar with the data, creating primary codes, searching for semantic units in the text, reviewing semantic units, defining and naming semantic units, and reporting. The management strategies of conflicts of interest in pharmaceutical sectors were determined as the sub-themes, and the overlapping themes were integrated. The data were synthesized in MS Word 2016.
4. Results
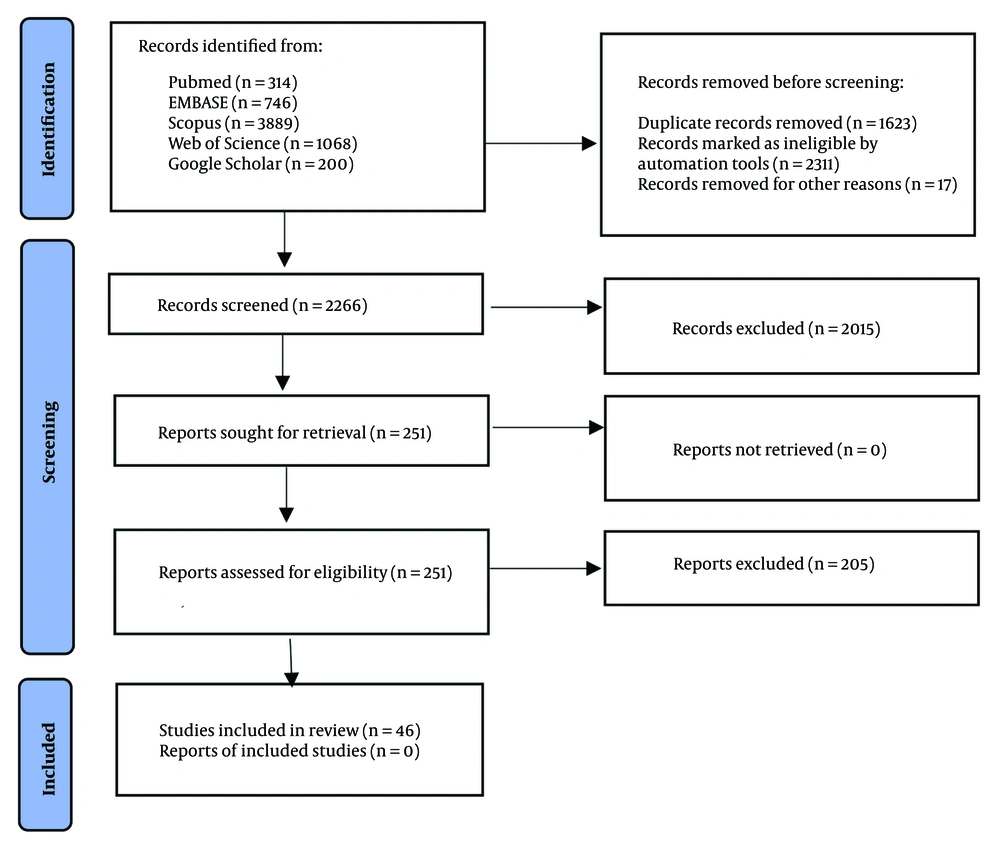
Figure 1 shows the process of including studies.
A total of 6,217 records were retrieved from the selected databases, out of which 46 records were deemed eligible for inclusion in the study (Figure 1). Table 1 presents the main characteristics of the included studies.
| Number | Author, Year, Country | Aim | Sample | Data Collection Tool | Solutions for Managing the Conflict of Interest |
|---|---|---|---|---|---|
| 1 | Miller et al. 2024. USA (23) | Assessing the characteristics and financial conflicts of interest of presenters, panelists, and moderators at hematology and oncology workshops held jointly with or hosted by the US FDA. | Information on all publicly available hematology or oncology FDA workshop agendas held between 1 January 2018 and 31 December 2022. | NA | Need for clear disclosures. More balanced selection of presenters with fewer conflicts may help to limit bias in discussions between multiple stakeholders. |
| 2 | Grundy et al. 2022. South-East Asia Region (24) | Determining the characteristics; and range of conflict of interest policy development in the region | Eleven countries in the WHO SEAR | Interview | Process for managing the conflicts of interest; recuse committee members with a conflict of interest from relevant work. Committee members should divest or otherwise be free from conflicts of interest. |
| 3 | Fabbri et al. 2022. Scandinavia (25) | Analyze the characteristics of conflict of interest policies at; Scandinavian medical schools | Scandinavian medical schools | NA | One or more schools had a restrictive policy. None of the schools had a restrictive policy for the five additional items (sales representatives, speaking relationships, on-site education activities, drug samples, and medical school curriculum). |
| 4 | Lau et al. 2018. Australia (26) | Investigate the relationship between health consumer organizations and the pharmaceutical industry and how to manage it in Australia | Random selection of 133 health consumer organizations | Extraction form | Disclosure of industry funding; disclosure of board members' employment information. Organizational policies for conflict of interest, advertising, and pharmaceutical companies funding. |
| 5 | Grundy et al. 2018. USA, France, Australia (27) | Comparing conflict of interest policy among the USA, France, and Australia | Primary and most recent sources of the transparency policies in each jurisdiction | Extraction form | Strengthening regulatory structures and promoting independence from the industry. Prohibiting certain relationships between health professionals and industry. Sunshine policies in the US, France and Australia all share the aim of making the ties between individual health professionals and health-related industries publicly transparent; informed consumers could address conflicts of interest. |
| 6 | Fabbri et al, 2018, EU (28) | Examine rules covering disclosure by pharmaceutical companies of their payments to health professionals in different European countries | Nine European Union countries | NA | France adopted a “Sunshine Policy” in 2011. Portugal and Latvia adopted laws mandating public disclosure in 2013 and 2014, respectively. Five other included countries (Italy, Germany, Spain, Sweden, UK) have adopted a self-regulatory approach; Netherlands has a mixed self-regulatory system with some government involvement. |
| 7 | Bélisle-Pipon et al. 2018, Canada (29) | Analyze conflict of interest disclosures of members of Québec’s immunization EAC | All public reports of the committee | Checklist | Clear and detailed disclosure of conflicts of interest by experts making all COI information publicly accessible. |
| 8 | Rhéaume et al. 2018; Canada (30) | Determine the existence and the level of healthcare professional; (HCP) knowledge of local policies regarding conflict of interest | All HCPs in the FMTUs authorized to hand out drug samples | Questionnaire | Only one-quarter of the FMTU directors reported having a policy regarding the relationship between the pharmaceutical industry and residents; education about pharmaceutical marketing practices |
| 9 | Young et al. 2018; USA (31) | Assesses the physician-industry conflict of interest disclosure database in the United States | One thousand five US residents | Questionnaire | Need for databases of disclosure information. Improve the quality and accessibility of this information. |
| 10 | Nissanholtz-Gannot et al. 2017, Israel (32) | Examine if the law of disclosure impacted the relationship between physicians in the Israeli health system and the pharmaceutical industry | Forty-six representatives of relevant stakeholders | Interview | Transparency of the relationship between PCs and physicians; regulate the relationship between physicians and PCs; Self-regulation |
| 11 | King and Bearman 2017. USA (33) | Examine the relationship between gift regulation and the diffusion of four newly marketed medications. | Using a dataset that captures 189 million psychotropic prescriptions written between 2005 and 2009 | NA | Existence of marketing regulation affects the use of new drugs. Gifts restrictions. Disclosure policies |
| 12 | Grundy et al. 2017. Australia (16) | Develop a deeper understanding of how those responsible for developing and implementing policy in the pharmaceutical industry conceptualize conflict of interest. | Ten past or current employees of pharmaceutical companies | Interview | Creating informed consumers. Proscribing or limiting problematic interactions or activities; making conflicts of interest visible so that they can be managed. Educating medical professionals about how to recognize and manage their conflicts of interest. Relying on industry to self-regulate. |
| 13 | Cosgrove et al. 2017. USA (34) | Assess (a) the disclosure requirements of GDGs in a cross-section of guidelines for major depression; (b) the extent and type of conflicts of panel members | Fourteen guidelines with a total of 172 panel; members | Extraction form | Limiting panel members to participate in industry speaker bureaus. Members of the [guideline development group] should divest themselves of financial investments they or their family members have, and not participate in marketing activities. |
| 14 | Ammous et al. 2017. Lebanon (35) | Assess the awareness and attitudes of the general public in Lebanon regarding the; Interactions between physicians and pharmaceutical companies | Two hundred sixty-three participants | Questionnaire | There is a definite need to raise awareness among the Lebanese population about the potentially negative impacts of physician–industry interactions on the quality and cost of their healthcare. There is a need for regulation physician–industry interactions. These may include self-regulation and governmental regulations. |
| 15 | Agarwal et al. 2017. India (36) | Investigate the various kinds of promotional methods employed by pharmaceutical companies in India and identify the potential harms of such techniques | NA | NA | Self-regulating and governmental regulations can reduce undue influence. |
| 16 | Chappell et al. 2016. Canada (37) | Examine the adequacy of existing academic conflict of interest rules. | Alzheimer’s drug therapy initiative in British Columbia | Interview | The majority of researchers perceive the influence of pharmaceutical manufacturers as problematic. Even when the strictest conflict of interest rules are followed. |
| 17 | Sierles et al. 2015. USA (38) | Ascertain whether changes occurred in medical student exposure to and attitudes about drug company interactions from 2003 - 2012 | One thousand two hundred sixty-nine third-year students at eight U.S. medical schools | Questionnaire | Medical schools and residency education should establish learning objectives and educate trainees about drug-company-sponsored research and marketing industry–physician and industry–trainee interactions, conflict of interest, and methods of reducing conflict of interest. Medical schools and residency programs should establish restrictive policies about industry–physician and industry-trainee interactions. |
| 18 | Dowers et al. 2015. USA (39) | Examines the implementation of a COI policy and related instructional activities at one veterinary college in the US. | Four hundred five students in the veterinary medical program | Questionnaire | Educational programming about pharmaceutical industry marketing practices can positively influence students’ attitudes toward drug companies and their representatives. |
| 19 | Kamal et al. 2015; Egypt (40) | Explore the perceptions of physicians towards promotional and marketing activities of pharmaceutical companies among physicians and pharmacists in Egypt | Nineteen physicians, dentists, pharmacists and policymakers | Interview | There was a need for more monitoring and regulation of pharmaceutical promotion. Monitoring, control and transparency of information on medicine efficacy |
| 20 | Pham-Kanter et al. 2014; USA (41) | Examine the relationship between the financial interests of FDA CDER advisory committee members and whether members voted in a way favorable to these interests. | Data set of voting behavior and reported financial; interests of 1,379 FDA advisory committee members | NA | Excluding individuals with certain kinds of ties from voting or participating may be an expeditious way to limit this bias. |
| 21 | Montastruc et al. 2014. France (42) | Describe the exposure and attitudes of French medical residents towards the pharmaceutical industry. | Residents from 6 French medical faculties | Questionnaire | Educate medical students about relationships with industry and conflict of interest. Create guidelines for the relationships with industry. Improve the culture of disclosure of conflicts of interest and transparency among residents. |
| 22 | Jahnke et al. 2014. Germany (43) | Assess the frequency and places of contact of German medical students to pharmaceutical promotion and examine their attitudes. | All clinical students at the University of Goettingen Medical School | Questionnaire | Contact between medical students and the pharmaceutical industry should be prohibited; Need for education of physicians and medical students about interacting with the industry. |
| 23 | Larkin et al. 2014. USA (44) | Estimate the effect of anti-detailing policies on off-label prescribing of antidepressants and antipsychotics by pediatricians | Data from 31; geographically diverse AMCs and their affiliated hospitals | NA | Introduction of strict detailing policies reduced prescribing among physicians serving pediatric patients of drugs marketed to physicians in detailing visits; bans or limits on gift giving by pharmaceutical sales representatives. Restrictions on sales representatives’ access to physicians. |
| 24 | Nguyen and Bero. 2013. USA (45) | Describe the content of Medicaid drug selection committees’ COI policies for the US states and the District of Colombia, categorize the policies by strength, and identify characteristics of a strong policy. | Official Medicaid websites and contact Medicaid staff by e-mail and/or telephone to identify drug selection committee COI | ||
| Policies | NA | The most common management strategy was disclosure of COI and then self-regulation. | |||
| 25 | King et al. 2013. USA (46) | Examine the effect of an active policy on restricting gifts from representatives of pharmaceutical and device industries on subsequent prescribing behavior | Fourteen US medical schools with an active gift restriction policy | NA | Exposure to a gift restriction policy during medical school was associated with reduced prescribing of two out of three newly introduced psychotropic medications. |
| 26 | Hughes and Williams. 2013. Canada (47) | Examine the ethical issues raised by such financial relationships in the context of drug; reimbursement decision-making | Quebec example of Coalition Priorate Cancer (CPC), a Quebec-based patient interest group | NA | In order to manage the COI, these groups should be; Disclose donors’ names publicly, as well as the amount, the nature, and the use of the support they receive. General donation should be preferred. Increase the role of advocacy groups without industry funding. |
| 27 | Epstein et al. 2013. USA (48) | Determine whether exposure to COI policies during psychiatry residency training affects psychiatrists’ antidepressant prescribing patterns after graduation | National administrative prescribing data from IMS Health for 1652 psychiatrists | NA | COI policies can help inoculate physicians against persuasive aspects of pharmaceutical promotion. |
| 28 | Chimonas, S. et al. 2013. USA (49) | Follow-up study in 2011 to assess possible improvements in medical schools’ management of COI | The Websites of all 133 medical schools existed in July 2011. | NA | Eliminate industry gifts, meals, and ghostwriting. Prohibit or “strongly discourage” speakers’ bureaus. Establish central repositories for product samples and industry funds for continuing medical education (CME), scholarships, fellowships, and travel. Require that members of pharmacy and therapeutics (P&T) and other purchasing committees be free of COI. Require full transparency for industry honoraria and consulting contracts. |
| 29 | Austad et al. 2013. USA (50) | Assess interactions between trainees and the pharmaceutical industry | First and fourth-year medical students and third-year residents, stratified by medical school | Questionnaire | During medical education, trainees gain the impression that they can resist undue influence from industry interactions. Policy changes at academic medical centers have recently been enacted to limit trainees’ interactions with industry. |
| 30 | Al-Areefi et al. 2013. Yemen (51) | Explore physicians’ attitudes about interactions with medical representatives and their reasons for accepting the medical representatives’ visits | Thirty-two physicians from both private and public hospitals | Interview | Monitoring promotional activities. Educational interventions concerning pharmaceutical marketing. Develop a suitable policy and regulations in terms of drug promotion. |
| 31 | Hutchins et al. 2012. USA (52) | Examine the American Academy of Neurology’s prevention and limitation of conflict of interest relationships with the pharmaceutical and medical device industry | AANs polices governing its interactions with industry | NA | The AANs Policy on conflicts of interest provides 4 mechanisms for addressing COI: Avoidance, separation, disclosure, and regulation. |
| 32 | Alssageer et al. 2012; Libya (53) | Examine the frequency of pharmaceutical company representative interactions with physicians in Libya. | One thousand Libyan physicians in selected public and private practice settings | Questionnaire | Restrictions on receiving free drug samples. Provide independent drug information. |
| 33 | Mason and Tattersall 2011. Australia (54) | Examine the adequacy of policies at Australian medical schools for managing potential conflicts of interest with the pharmaceutical industry | Twenty Australian medical schools | AMSA Pharm Free Scorecard | Prohibition of giving gifts to students by industry. Restrictions on communication with industry. Disclosure of communications with industry. Educate students about relationships with industry and conflict of interest. |
| 34 | Chimonas et al. 2011. USA (55) | Determine the extent and strength of medical schools’ CCOI policies at U.S. medical schools | Compliance officers at 125 MD-granting medical schools in the United States | Questionnaire | Full disclosure of all links with industry and conflict of interest. Making disclosure information available on websites. Educate employees about communication with the industry. |
| 35 | Lexchin et al. 2010; EU (56) | Investigate the normative character of the COI policies and practices of 3 drug regulatory agencies in Europe | NA | NA | Disclosure of communications of members of regulatory agencies and the pharmaceutical industry. Accessibility of transparency information. |
| 36 | Cosgrove and Bursztajn 2010. USA (57) | Analyze the gaps in existing COI policies | NA | NA | Educate prescribing clinicians about the importance of disclosing their potential COI. Report all financial relationships with pharmaceutical companies and medical device manufacturers; Potential COI should be made available to all parties. |
| 37 | Campbell et al. 2010. USA (58) | Estimate the nature, extent, consequences, and changes in PIRs nationally | Random sample of 2938 primary care physicians and specialists | Questionnaire | Banning certain types of PIRs, such as drug samples and industry-sponsored meals and participation in speaker bureaus |
| 38 | Bruyere et al. 2010. EU (59) | Review potential problems in the relationship between academics and the industry | Academic experts and members of the pharmaceutical industry | Expert consensus meeting | Individuals who have competing interests generally should be excluded from voting; guidelines for a transparent, ethical, strong, and successful partnership between the academic scientist and the pharmaceutical industry have been provided. |
| 39 | Hartung et all. 2010; USA (60) | Evaluate the effect of a policy prohibiting prescription drug samples and pharmaceutical industry interaction on prescribing patterns in a rural family practice clinic in central Oregon | NA | NA | Prohibiting prescription drug samples and pharmaceutical industry interaction. Restricting access of pharmaceutical sales representatives to the MMG family practice clinics. Elimination of drug samples. |
| 40 | Anderson et al. 2009; USA (61) | Examine relationships between pharmaceutical representatives and; obstetrician–gynecologists | Five hundred fifteen randomly selected physicians in the American College of Obstetricians and Gynecologists’ Collaborative Ambulatory Research Network. | Questionnaire | Use guidelines on relationships with the industry. |
| 41 | Newcombe and Kerridge. 2007. Australia (62) | Understanding of how HREC approaches the problem of potential conflicts of interest arising from pharmaceutical sponsorship of clinical research | HREC chairpersons in New South Wales (N=27) | Questionnaire | Disclosure of researchers' relationship with industry. Advice to researchers to limit their involvement with the trial sponsored by the pharmaceutical-industry or divest themselves of the relationship with the industry. |
| 42 | Chimonas et al. 2007. USA (63) | Determine physicians’ techniques for managing cognitive inconsistencies within their relationships with drug representatives | Thirty-two academic and community physicians in San Diego, Atlanta, and Chicago | Focus group | Prohibition of physician–detailer interactions. Disclosure of contracts between industry and physicians. Gift-giving limitation. |
| 43 | Cosgrove et al. 2006. USA (64) | Examine the degree and type of financial ties to the pharmaceutical industry of panel members responsible for revisions of the diagnostic and statistical manual of mental disorders | One hundred seventy panel members who contributed to the diagnostic criteria produced for the DSM-IV and the DSM-IV-TR. | NA | The APA institute a disclosure policy for panel members of the DSM who have financial ties to the drug industry. |
| 44 | Ball et al. 2006. UK (65) | Examine advertising and disclosure of financial support by pharmaceutical companies on the websites of major patient organizations | Sixty-nine national and international patient organizations | NA | Full disclosure of all pharm donors and the nature and amounts of the donations; develop an ethical code of practice to guide relations with pharm; Declare any possible conflicts of interest to the organization’s trustees and on the website. |
| 45 | Keim et al. 2004. USA (66) | Examine the beliefs and practices of emergency medicine program directors regarding interactions with the pharmaceutical industry | The Board of the Council of Emergency Medicine Residency Directors | Questionnaire | Restrictions on the relationship between students and industry. Restrictions on the distribution of free drug samples. |
| 46 | Choudhry et al. 2002. Canada (67) | Quantify the extent and nature of interactions between authors of CPGs and the pharmaceutical industry | One hundred ninety-two authors of 44 CPGs endorsed by North American and European societies | Questionnaire | Authors who have significant conflicts of interest should be excluded from participating in the guideline creation process; authors must disclose relationships with the pharmaceutical industry before guideline meetings are held. |
Characteristics of the Included Studies
Based on Table 1, most of the articles were published in the USA (23 records), followed by Australia (5 records), Canada (5 records), Lebanon (4 records), the EU (3 records), France (2 records), and Scandinavia, Southeast Asia, Israel, India, Egypt, Germany, Yemen, Libya, and the UK, each with 1 record. These articles were published in various years: 2024 (1 record), 2022 (2 records), 2018 (7 records), 2017 (7 records), 2016 (1 record), 2015 (3 records), 2014 (5 records), 2013 (7 records), 2012 (2 records), 2011 (2 records), 2010 (5 records), 2009 (1 record), 2007 (2 records), 2006 (2 records), 2004 (1 record), and 2002 (1 record).
Data collection methods varied among the studies: Interviews (6 records), extraction forms (5 records), questionnaires (15 records), checklists (1 record), AMSA Pharm Free scorecard (1 record), expert consensus meetings (1 record), focus groups (1 record), and 19 documents did not mention any data collection tool. The quality assessment of the included articles is provided in Appendix 1 in Supplementary File.
Table 2 outlines the effective strategies for managing conflicts of interest, categorized into three main themes: Industry relationship management, empowerment, and transparency and disclosure. These strategies are supported by two mechanisms: Legislation and self-regulation.
| Category | Sub-category |
|---|---|
| Industry relationship management | Restrictions on the relationship between employees and industry; creating guidelines for interacting with industry; revolving doors; divestiture of the right to voting; managing patient advocacy relationships with industry; continuing medical education sponsorship limit |
| Empowerment | Education; create an independent drug database |
| Transparency and disclosure | Transparency rules; transparency of communication with the industry; standardization of transparency reports |
| Support mechanisms | - |
| Government regulation and policies | - |
| Self-regulation | - |
Strategies of Management of Conflict of Interest
4.1. Industry Relationship Management
4.1.1. Restrictions on the Relationship Between Employees and the Industry
Most healthcare providers rely on academic education, lectures, permits, study results, and medical guidelines for their remedies and therapies. Therefore, the content of these resources should be strictly based on academic evidence, free from any bias caused by conflicts of interest. Pharmaceutical industries and their representatives often interact with physicians, healthcare employees, and key influencers within healthcare systems. They attempt to create a favorable image of themselves and influence decisions by offering various incentives and gifts, such as free drug samples, meals, travel tickets, and tickets to scientific conferences (68).
Studies on industry relationship management have examined prohibiting or restricting relationships between physicians (15, 28, 32, 58), medical students (38, 48, 50, 66), lecturers (34), members of the Committee on Selection and Use of Medicines (49), researchers (62), guideline developers (67), and the industry. Managing gifts and incentives through the control and restriction of gifts from the pharmaceutical industry (27, 46) and gifts received by individuals (36, 60) are also considered.
To prevent the adverse effects of industry influence on guideline developers, the Institute of Medicine (IOM) has recommended that members of guideline development groups avoid financial investments for themselves or their family members and refrain from participating in marketing activities or advisory boards of companies whose interests could be affected by these guidelines (34).
Since conflicts of interest typically arise when individuals and the industry interact, reducing these interactions by restricting relationships between the industry and individuals can significantly lower the chances of such conflicts. Limiting interactions between physicians and the industry can positively influence physicians' prescribing behavior (69). At the organizational level, this goal can be achieved by restricting free drug samples, advertising goods, and meetings with pharmaceutical company representatives. For example, Stanford University has prohibited its hospitals from directly interacting with drug sales agents. At higher levels, policymakers may adopt laws to regulate the permissibility of such interactions. For example, Minnesota has enacted a law prohibiting the pharmaceutical industry from giving gifts exceeding 50$ per year (15).
4.1.2. Creating Guidelines for Interacting with the Industry
Healthcare providers often interact with industry representatives who visit for promotional purposes. Developing guidelines that outline appropriate ways to interact with the industry can help improve employees' behavior towards the industry and reduce its influence on them (53). For example, the American Medical Association (AMA) and the American College of Obstetricians and Gynecologists (ACOG) have developed guidelines on industry gifts to physicians or industry relationships (61).
4.1.3. Revolving Doors
Another aspect of industry relationship management is controlling job transfers between the private and public sectors. For instance, in England, individuals seeking positions must first fill out specific forms detailing their previous or future roles in the industry, which are then reviewed before approval (56).
4.1.4. Divestiture of the Right to Vote
Conflicts of interest can be mitigated by restricting the presence of individuals with conflicts of interest and prohibiting them from participating in discussions or voting in meetings (24, 25, 29, 41, 45, 59). For example, in the U.S., one strategy against conflicts of interest in the Committee on Selection and Use of Medicines is to prohibit or restrict the participation of members with conflicts of interest in these committees, which varies among different states (45).
4.1.5. Management of Patient Advocacy Relationships with Industry
Some organizations in many countries help patients receive medical care and counseling. Recognizing the impact of these organizations on patients and the services provided, many pharmaceutical companies and industries attempt to interact with these organizations or their employees and offer financial aid and gifts. Thus, creating and enforcing ethical codes that govern relationships between these organizations and the industry would be highly beneficial (65).
4.1.6. Continuing Medical Education Sponsorship Limit
Providing continuous education to individuals is a powerful promotional tool to encourage the prescription of industry drugs. The entry of industry funds into education and the dependence on these funds can adversely affect the content and quality of education, favoring the industry. Limiting the sponsorship of continuing medical education by the pharmaceutical industry is another strategy used to manage conflicts of interest through legislation (27, 49, 54).
4.2. Empowerment
Most conflicts of interest arise from relationships between the industry and individuals in the healthcare sector. By interacting with healthcare providers, offering incentives and gifts, the pharmaceutical industry and its agents attempt to create secondary interests aligned with their own, potentially altering healthcare providers' decisions. Therefore, it is crucial to empower individuals to resist the influence of the pharmaceutical industry or to reduce the need to interact with it.
4.2.1. Education
Universities train the next generation of health professionals and employees. In contrast, the drug industry and its sales agents focus significantly on medical students as their target audience, aiming to influence their decisions, particularly their future prescriptions. Therefore, including courses on managing conflicts of interest and relationships in curricula can inform and prepare students for potential conflicts of interest situations and teach them to respond appropriately. This education helps prepare the next generation of professionals against the harmful effects of conflicts of interest (50).
Although most students believe that receiving gifts from the industry is not problematic and that such gifts will not negatively affect their future prescriptions (39), numerous studies have documented the influence of education on students' attitudes and behavior and their ability to resist industry influence (15, 30, 38, 39, 43, 54, 55, 70). This education should enable students to learn how to identify, evaluate, and manage potential conflicts of interest to minimize the impact of such situations and be well-prepared to handle them (37, 70).
Education is not only for students; all healthcare system employees must also be appropriately prepared and have the right attitudes toward dealing with conflicts of interest. According to Al-Areefi et al., while some physicians have a negative attitude towards pharmaceutical company agents and their behaviors, considering them unethical, they are still willing to meet with these agents and see receiving free drug samples and gifts as normal and ethical (51). This indicates that even physicians should be prepared to handle conflicts of interest and the harmful effects of relationships with the industry. Because some individuals mistakenly trust the industry and/or underestimate the industry's influence, they may be unconsciously affected. Furthermore, these individuals may not respond appropriately when facing conflicts of interest due to a lack of proper education. Therefore, continuous in-service training programs are necessary for physicians and employees. When physicians are well-prepared to cope with the industry's effects and conflicts of interest, they can minimize the industry's impact on their profession accordingly. Professional organizations play a critical role in preparing physicians and maintaining public trust in this profession (69).
In addition, educating and empowering patients and the public about the relationship between the pharmaceutical industry and healthcare providers can increase their awareness and sensitivity to these relationships (35, 57, 58). This education enables people to demand transparency and anti-corruption measures and prevents them from easily prioritizing personal interests over public interests in conflict of interest situations.
4.2.2. Creating an Independent Drug Database
In some cases, physicians must inevitably meet with drug agents due to their need for information in certain situations. Indeed, one reason physicians have cited in studies for accepting meetings with pharmaceutical agents is limited access to pharmaceutical information (53). Pharmaceutical company agents initially interact with physicians to provide the latest information on new drugs while offering gifts, incentives, and other proposals (51). Therefore, creating pharmaceutical databases and programs that provide the data physicians require and similar solutions can reduce physicians' need for these data and interactions with agents and the industry (15, 42, 70). When the demand for relationships between physicians and the industry is reduced, financial and non-financial incentives are less likely to be offered to physicians. Hence, physicians are less likely to be biased towards the interests of the pharmaceutical industry.
4.3. Transparency and Disclosure
Transparency of financial and non-financial relationships: Transparency of financial and non-financial relationships is crucial in managing conflicts of interest in the pharmaceutical sector. Transparency in individuals' and organizations' activities prevents potential violations and corruption. This way, the public will always oversee the activities and measures taken. The first step to managing conflicts of interest is transparency and disclosure (47).
4.3.1. Transparency Rules
Studies have highlighted the importance of transparency. It is crucial to use legislative power to support transparency policies so that transparency becomes a legal and mandatory issue. In most countries, transparency laws are compulsory. For example, the Sunshine Act in the USA requires drug and medical equipment manufacturers to make their contracts with health professionals and hospitals publicly accessible (36, 69). Similar laws have been adopted in many other countries, including some European nations (27, 28). Evidence suggests that adopting transparency rules has reduced the prescription of new drugs (33). Thus, transparency can be seen as a powerful tool for managing conflicts of interest in the pharmaceutical sector, helping to prevent the industry's adverse effects and protect public trust.
4.3.2. Transparency of Communication with the Industry
Industry relationships with healthcare professionals are necessary or useful in many cases. Therefore, it may be unreasonable to restrict or prohibit these relationships entirely. However, transparency can play a vital role in managing the effects of these relationships and conflicts of interest and preventing unhealthy relationships. It can also increase public awareness and trust in valuable and constructive relationships (27). Industry relationships with health professionals (70), guideline developers (67), researchers (57), non-government organizations (NGOs) (26), universities of medical sciences (54), members of the Committee on Selection and Use of Medicines (45, 64), and members of regulatory agencies (56) should be transparent. To enhance the efficiency of transparency, the transparency data should be entirely open to public observation so that everyone can easily access complete and practical data, contracts, gifts, and relationships between the pharmaceutical industry and the healthcare system (29, 55, 65). For example, all research institutions in the USA must maintain a daybook for sponsored research containing all accessible data on industry funding directly and indirectly registered (57).
4.3.3. Standardization of Transparency Reports
Transparency reports should be standardized (28) so that all details are accessible consistently in all cases. These can cover the amount and value of gifts, the dates they were received (55), the percentage of individuals' income arising from the pharmaceutical industry, and other information about communications and contracts between the industry and health professionals (65). Publishing these data in analyzable formats allows the public to determine the extent of health professionals' dependence on the industry and even the correlation between their decisions and the level of dependence (29). A European study revealed that data searchable and extractable for analysis is only available in England. Conversely, data were searchable in France, Holland, and Portugal but could not be extracted (28).
The above topics are the conflict of interest management solutions reported in the published articles. These solutions are executed through two mechanisms or systems below:
(1) Government regulations and policies: Using governance capacities in legislation, execution, and supervision of the solutions mentioned above is the first case. Governments must manage issues related to communication with the industry, empowerment, and transparency using the tools provided.
When all legislation capacities are utilized, the interests of all parties are considered, and the likelihood of conflicts of interest and their negative impacts is diminished (52). A lack of proper regulations in pharmaceutical marketing has been a significant issue in some pharmaceutical systems. Therefore, passing and enacting appropriate rules to enhance control over the pharmaceutical industry and its agents and diminish their impact on physicians' prescriptions is necessary. Numerous studies have shown that restricting advertising and marketing by the pharmaceutical industry through laws and regulations allows control over the industry's effects on health professionals (33, 44, 51, 63). For example, in a study in Yemen, it was found that establishing laws and policies for pharmaceutical advertising enhanced the ability to monitor the advertising activities of pharmaceutical companies and their agents and created a legal framework for marketing activities (51). Another study revealed that new drug dispensing is less common in states with marketing laws than in states without such regulations (33). This suggests that the pharmaceutical industry's influence on physicians' prescriptions through incentives and gifts is diminished when the legislative framework is improved, and appropriate marketing laws are enacted.
Additionally, creating mechanisms to monitor industry advertising activities and supervising individuals' activities and communications can take significant steps in managing these communications (40, 51, 57).
Despite the importance of legislation in managing conflicts of interest, comprehensive monitoring to identify conflict of interest cases and situations is also essential. These mechanisms allow for monitoring the activities of the pharmaceutical industry and individuals, as well as the relationship between them and the duties and interests of individuals, helping to identify conflict of interest situations and areas requiring legislation for better control (56).
(2) Industry self-regulation: Another mechanism for managing conflicts of interest is using the capacities of the industry itself. According to the literature, many countries use the capacities of the industry and organizations to manage conflicts of interest (16, 28, 35, 36, 54).
Most countries use legislation to control and manage relationships between the pharmaceutical industry and individuals and conflicts of interest. However, in many countries (e.g., Australia, the EU, Canada, and Japan), the industry is responsible for regulating relationships between the industry, individuals, and organizations (16).
A study by Fabbri et al. on nine European countries about disclosure policies revealed that five of nine countries adopt industry self-regulation policies. In these countries, the government is not responsible for disclosing policies; rather, the industries handle transparency (28). Industries can adopt ethical codes to avoid incentives given to health professionals and other advertisements used to influence them (36). This aligns with the social responsibilities of firms and industries for public health. Since one side of the conflict of interest situation always involves financial incentives from pharmaceutical firms and industries, and offering such incentives is a driver of secondary interests in individuals, it is vital to use the industry's capacity to reduce these incentives. Additionally, voluntary transparency and the use of ethical codes by the industry allow for managing conflicts of interest more efficiently and at lower costs.
5. Discussion
This study aimed to identify solutions for managing conflicts of interest in the pharmaceutical sector. As a result, 46 records were included in this systematic review. Except for a few articles from the Middle East (e.g., Lebanon, Yemen, and India), most studies were conducted in developed countries with advanced healthcare systems. This implies that being an advanced and developed nation influences the attention paid to conflicts of interest.
The parameters identified in this study for managing conflicts of interest in the pharmaceutical sector were managing communications with the industry, empowerment, transparency, and disclosure, supported by two mechanisms: Government legislation and industry self-regulation. Bahadori et al. (71) found similar results in their study.
Given the relationships between the industry and various parts of the pharmaceutical systems and recognizing that such communications can be helpful, critical, detrimental, or destructive, it is essential to manage these communications. Restricting or prohibiting communication, managing gifts and incentives from the industry, or finding solutions to reduce the effects of communications between individuals and organizations with the industry can resolve or reduce most conflicts of interest. When assessing the relationship between the industry and health professionals and those influential in the healthcare system, the goals of the parties are often contradictory: The industry aims to increase profits (72), while the healthcare system aims to improve public health. Since the industry will always try to influence healthcare employees in favor of its interests and goals, creating a conflict of interest, it is necessary to avoid direct communication between the industry and the policy-making and management levels of the healthcare sector. Therefore, more restrictions and control over these communications will reduce the likelihood of such harmful effects.
Additionally, because industry communication with individuals and organizations in the pharmaceutical system is often unavoidable, empowering human resources to manage such communications and resulting conflicts of interest is crucial. Given the industry's efforts to influence healthcare providers, conflicts of interest can be effectively managed if employees are well-trained in university or through regular in-service courses against the industry and conflicts of interest, are prepared, and develop the appropriate attitudes and cultures about this phenomenon.
Finally, transparency and disclosure are significant solutions for managing conflicts of interest mentioned in the literature. Public access to data on financial and non-financial activities, gifts paid and received, and individuals' decisions and voting results creates a form of public supervision over these issues. As the public becomes aware of this data, people can act as supervisors and achieve decentralized supervision. Since people are more concerned about their lives than regulatory bodies, they will attempt to monitor issues more effectively (27). However, decentralized supervision should not replace centralized supervision; instead, these two should complement each other to enhance public trust in the pharmaceutical system (31).
All the solutions mentioned above for managing conflicts of interest require a mechanism to work efficiently. Governments can be effective if they use their power to regulate relationships, empower individuals, and enforce transparency using relevant regulations and policies. Additionally, the industry's capacity can be leveraged to implement policies against conflicts of interest. In this way, the industry will be responsible for implementing policies on conflict of interest management, such as transparency or refraining from giving incentives to employees. Since gifts and incentives are offered by the industry, the industry's commitment to conflict of interest management policies and self-regulation is expected to enhance the effectiveness of these programs.
5.1. Limitations
One limitation of this review was the lack of access to full texts of numerous qualified articles. Additionally, most studies were conducted using survey and cross-sectional methods that did not mention management solutions for conflicts of interest in the pharmaceutical industry. Furthermore, there was no access to related medical databases such as CINAHL, which may contain related articles. Therefore, it is suggested to investigate and introduce recent and comprehensive policies in each dimension mentioned in this study based on new conditions.
5.2. Conclusions
This systematic review revealed that conflict of interest in the pharmaceutical sector is managed through communication management, empowerment, education, and, most importantly, transparency in financial and non-financial relationships, supported by government regulations or industry self-regulation. These measures must be implemented and organized into a coordinated and integrated system.