1. Background
About 80% of communicable diseases around the world are waterborne (1, 2). Access to improved drinking water is unavailable to an estimated 884 million people around the world, most of which live in rural, dispersed, and often remote communities in developing countries (3).
There are many pollutants in water, such as pathogenic organisms, fecal matter, suspended solids, algae, organic matter, and harmful chemicals. Among the various adverse pollutants, coliform bacteria and arsenic are very important (4, 5). Coliform bacteria are the indicator of water contaminated with human or animal wastes and if these exist in water, it is unsafe for drinking purposes. Generally, all bacteria are not harmful but other microbes could cause short-term health effects, such as diarrhea, cramps, nausea, headaches, or other symptoms (5, 6).
Arsenic (As) contamination in groundwater, used for drinking purposes, has been envisaged as a problem of global concern (7). Elevated arsenic concentration in drinking water sources is an issue of global concern and threatens over 200 million people worldwide, especially in Asia. Arsenic has been reported in groundwater of Bangladesh, Cambodia, China, Taiwan, Mongolia, India, Japan, Myanmar, Nepal, Pakistan, Thailand, Viet Nam, and Iran (8-10). In the rural areas of west and northwest Iran, Kurdistan and Azerbaijan provinces, arsenic contamination of groundwater was reported (9). Arsenic exists in multiple oxidation states (+5, +3, 0 and -3); arsenate As (V) and arsenite As (III) are the most common inorganic forms of arsenic in aquatic environments. Arsenate species (AsO43-, HAsO42-, and H2AsO4-) are considered as soft acid and mostly stable in oxygen rich environments. However, arsenite species (AsO33-, AsO2OH2-, As (OH)4- and As(OH)3) are stable in moderate reducing environments, such as underground water. Furthermore, As (III) has higher toxicity and greater mobility, which needs to be oxidized for better adsorbption of As (V) (8, 11).
Arsenic in aqueous systems can originate from natural sources (e.g., geochemical reactions and volcanic emissions) as well as anthropogenic activities (such as metal mining, industrial waste discharge, and agricultural use of arsenical pesticides) (12). These sources could pollute water systems, especially groundwater aquifers from different sources. Drinking of arsenic-contaminated water has become a serious threat to public health, and has affected millions of people across the world (Kong et al. 2014). Ingestion of inorganic arsenic could result in both cancer (skin, lung, and urinary bladder) and non-cancer effects (13). Long-term exposure to high levels of arsenic may cause serious health problems, including skin, cardiovascular, neurological, renal, and respiratory diseases in humans (8, 14). To reduce the incidence of waterborne diseases and make the water suitable for human consumption, the removal of water pollutants are absolutely necessary (4, 15).
The world health organization (WHO) has set guidelines of 0.01 mg/L and 0 MPN/100 mL for arsenic and coliform bacteria in drinking water, respectively (16).
Different treatment technologies to reduce concentrations of arsenic in drinking water are available or under investigation. Some of these include coagulation (17, 18), sedimentation-filtration (19, 20), nanofiltration (21, 22), reverse osmosis (21, 23), fluidized-bed sand reactor, and subsurface groundwater treatment (24). Nevertheless, these technologies are inappropriate for application in rural communities (25). Therefore, in these communities, simplistic design, and minimum maintenance and operating cost are some important factors that require consideration (26). More than 50 household treatment technologies exist worldwide for water pollution removal (24). Arsenic removal by low-cost adsorbents, such as filter based granulated adsorbents, has been the most promising technique, which meets all the mentioned criteria offering reliable and efficient performance for communities living in scattered settlements (8, 25, 27). However, natural adsorbents are favorable for their low-cost and abundant sources, yet, some studies have shown that they had no sufficient capacity to remove total arsenic (As (III) + As (V)) from water resources (28, 29). For example, natural adsorbents, such as limestone, and zeolites like clinoptilolite, chabazite, and sandy soils have been studied for arsenic removal in water (30). The acceptable level of concentration of arsenic in drinking water is 0.01 mg/L (31). Pravin et al. in 2009 used conventional and modified filters to remove coliform bacteria and arsenic. The study results showed that the efficiency of conventional and modified filters to remove coliform bacteria were 99.95% and 99.99%, and in the removal of arsenic were 14% and 75%, respectively (6). The study results of Aviles et al. in 2013 showed that the efficiency of the domestic filter in arsenic removal was 95.4% (initial concentration of 0.11 mg/L) (24). The considerable removal efficiencies of arsenic were also reported by several studies that used household filter, ceramic filter, and modified natural zeolite filter in Vietnam, Bangladesh, and Turkey (13, 32, 33).
A study, done in 2011, showed that adsorbent characterization of natural zeolites could be affected by parameters, such as their surface morphologies, chemical composition, physical properties, and specific surface areas (32). In another study in 2002, the researchers used granular slag columns for lead removal. It was concluded that the apparent mechanisms of lead removal by this column were sorption (ion exchange and adsorption) on the slag surface and precipitation (34).
2. Objectives
The aim of this study was the use of a native and low-cost absorbent (such as clinoptilolite) and unusable material (such as blast furnace slag), as a filter media, to remove arsenic and coliform bacteria from the water resource of small communities.
3. Methods
3.1. Filter Media Preparation
For filter media preparation, one of the essential stages is determination of solubility degree of filter media. This was determined in acidic media by dipping 10 g of each filter media in 32 mL of pure hydrochloric acid, which was mixed with 50% distilled water and was contacted for 30 minutes in the lab, with ambient temperature. The contacted filter media was rinsed, dried at 110°C, and weighed. Finally, the degree of solubility was calculated by dividing the weight lost to the initial weight and multiplication by 100. It should be noted that the dissolution rate of each filter media was not > 5% (35).
3.2. Experimental Setup
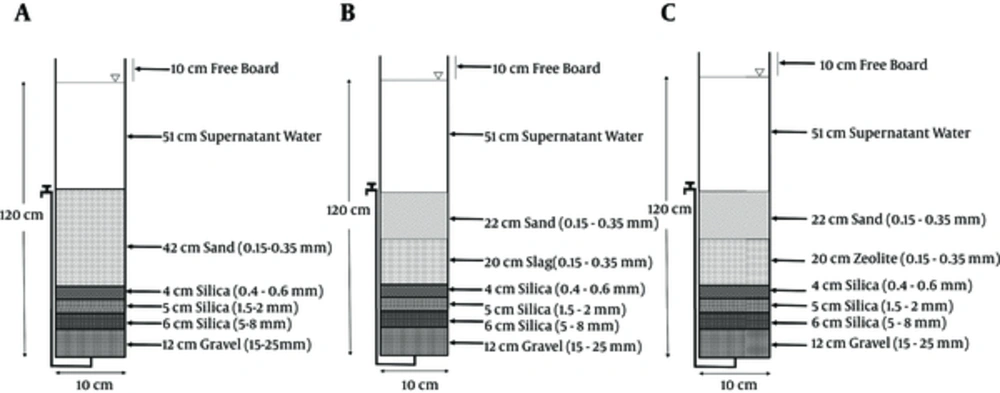
The experimental setup of this study is illustrated in Figure 1. As indicated, the pilot plant consisted of several parts, such as 1 water reservoir and 3 separate filters. Each filter had a 9-cm inner diameter and 120 cm height and was prepared from Plexiglas tubes. Other parts consisted of an electro-pump and hose connections. The conventional SSF media was modified by zeolite and blast furnace slag (BFS). The pilot plant consisted of 3 separate filters with different media, which were prepared according to AWWA standards (36).
The first set of filter [Figure 1A] similar to conventional slow sand filters (SSFs) was filled only by sand as filter media. It was maintained as a control.
The second set of filter [Figure 1B] was modified by replacing the 20-cm blast furnace slag (BFS).
The third set of filter [Figure 1C] was modified by replacing the 20-cm natural zeolite, clinoptilolite.
In this study, water resource for all study stages was tap water that was supplied from deep wells around Zayandehroud river, Isfahan, center of Iran. Filtration rate was adjusted between 0.1 and 0.24 m3/m2/h (37). Thus, the flow rate of each filter was 1.5 L/h. Arsenic in the forms of Na2HAsO4.7H2O and NaAsO2 was used for the preparation of stock synthetic solutions in distilled water (6, 38). Series of dilution were prepared from this stock in the range of 0.073, 0.11, 0.171, 0.21, 0.24 and 0.33 mg/L. Triplicate samples were taken after a 24-hour interval for each initial arsenic solution that was injected to the filters. The concentrations of arsenic were determined by ICP-AES (Model 2, Jobin Yvon ultima, France). The initial microbial stock solutions were prepared using diluted samples of Isfahan north wastewater treatment plant effluent and after samples were injected into the filters. Then, the total and fecal Coliforms were measured by multiple probable number (MPN) 9-tube method (39). Total hardness and turbidity of the samples were measured by titration with EDTA 0.01 M, and nephelometer (EUTECH TN-100), respectively (35). The cation exchange capacity (CEC) of filter media was measured by the methods used in previous studies (35, 40).
4. Results
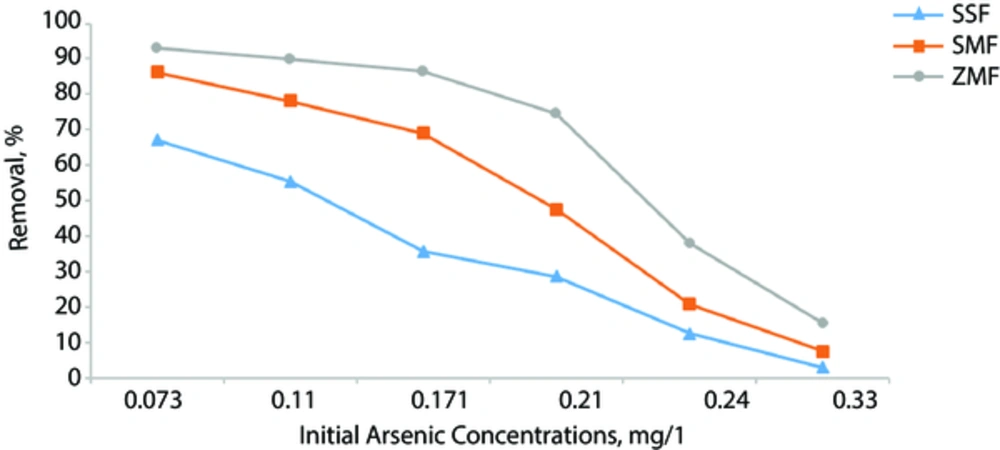
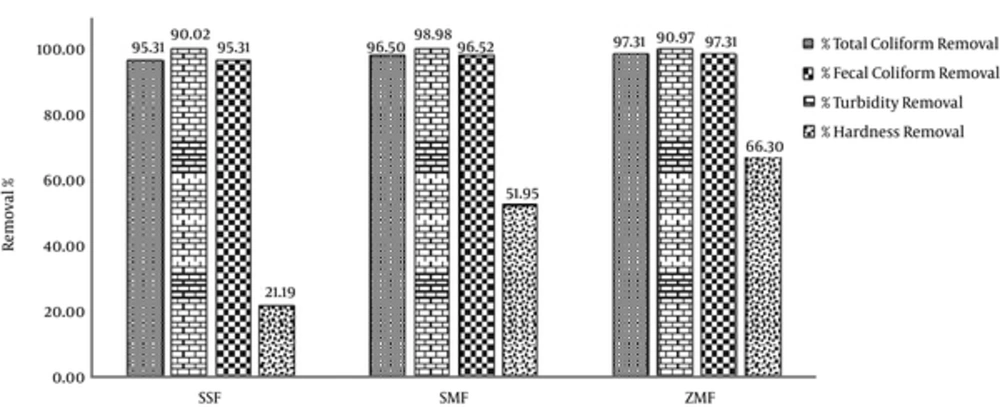
Table 1 illustrates the characteristics of the filter media used in conventional sand filter and modified filters by zeolite and slag. The characteristics of the water samples used for the experiments are shown in Table 2. Table 3 shows arsenic concentrations of influent, finished water and its removal efficiencies by SSF, SMF, and ZMF. The trend of arsenic removal efficiencies and total coliform, fecal coliforms, turbidity and hardness removal (%) by SSF, SMF, and ZMF are presented in Figure 2.
| Bed | Size, mm | Bed Weight, g | Density, kg/m3 |
|---|---|---|---|
| Sand | 0.15 - 0.35 | 2.17 | 2173.9 |
| Slag | 0.15 - 0.35 | 2.85 | 2857.1 |
| Zeolite | 0.15 - 0.35 | 1.53 | 1538.4 |
| Silica | 0.2 - 0.4 | 2.63 | 2631.6 |
| Silica | 0.5 - 0.8 | 2.73 | 2739.7 |
| Silica | 1.5 - 2 | 2.53 | 2531.6 |
| Gravel | 15 - 25 | 2.56 | 2564.1 |
Characteristics of the Filter Media Used in Conventional Sand Filter and Modified Filters by Zeolite and Slag
| Parameter | Mean ± SD |
|---|---|
| pH | 7.2 ± 0.13 |
| Temperature, °C | 23 ± 0.5 |
| Turbidity, NTU | 1 ± 0.5 |
| TDS, mg/L | 645.9 ± 277.8 |
| EC | 1030 ± 340.5 |
| Total Hardness, mg/L as CacO3 | 750 ± 406.6 |
| Alkalinity, mg/L as CacO3 | 163.1 ± 28.8 |
| Total coliform, MPN/100 mL | 4 × 106 ± 1428 |
| Fecal coliform, MPN/100 mL | 171.8 ± 115.5 |
| Arsenic, mg/L | 0.189 ± 0.054 |
Characterization of the Water Samples Used for the Experiments
Arsenic Concentrations of Influent, Finished Water and its Removal Efficiencies by SSF, SMF and ZMF
The mean removal efficiency of hardness, turbidity, and total coliforms by SSF, SMF, and ZMF are tabulated in Figures 3.
5. Discussion
Table 3 and Figure 2 show the trend of arsenic removal is the same in the three filter media. Therefore, in all influent water, the highest and the lowest removal efficiencies were obtained at low and high arsenic concentrations (0.073 and 0.33 mg/L), respectively. In other words, by increasing the influent arsenic concentrations, the arsenic removal efficiencies were decreased, so that, in the 0.33 mg/L arsenic concentration, the filters practically did not show high efficiency in arsenic removal. This may be due to the high initial concentration of arsenic, low area of filters, and saturation of media adsorption sites during operation of the filters Rany Devi et al. 2008, showed that by increasing treatment time, the adsorbent sites of the media would tend towards saturation (4). The highest removal efficiencies of arsenic that were obtained in a concentration of 0.073 mg/l by SSF, SMF, and ZMF, were 67.1%, 86.3%, and 93.1%, respectively. Overall, the mean removal efficiencies of SSF, SMF, and ZMF were 33.7%, 51.5%, and 66.2%, respectively. The priority removal efficiency of the 3 studied media is as follows: R ZMF > R SMF > R SSF.
In comparison, the studied filters indicated that SSF in any of the initial concentrations of arsenic couldn't meet the WHO guidelines. However, the SMF only in the initial concentration of 0.073 mg/L and the ZMF in concentrations of 0.073 and 0.11 mg/L could meet WHO guidelines. The corresponding removal efficiencies by ZMF were 93.1% and 90%, respectively. Nitzsche et al. in 2013 reported 96% arsenic removal for the initial concentration of 0.115 mg/L by using household filters (33). Most of these technologies could be used for As removal in large and medium scale treatment plants for centralized services, yet are not appropriate for rural areas where only untrained operators are available to install and maintain these technologies for domestic use. Other technologies accomplish similarly or even better outcomes regarding their As removal efficiencies, e.g., chemical coagulation or electrocoagulation (depending on As species up to 93% to 99% As removal efficiency (41) or pressure-driven membrane-based methods such as nano-filtration or reverse osmosis; both up to 99% arsenate removal efficiency. However, these techniques are much more expensive than the simple sand filter systems and have a high operation and maintenance cost. Some methods, such as oxidation treatments by ozone or coagulation–flocculation, require the addition of chemical compounds that produce toxic or carcinogenic by-products. Filters based on activated carbon are not as efficient regarding adsorption, having an AS removal efficiency of only up to 60% (42).
Although, all three filter media, SSF, ZMF, and SMF, had partial arsenic removal efficiencies and their mechanisms could be cation exchange capacity, adsorption, and precipitation, but among them, the ZMF showed the highest arsenic removal efficiency, due to having a high cation exchange capacity, sorption and precipitation more than slag and sand. The SMF also showed a satisfactory removal efficiency compared to the SSF, which may be due to adsorption, precipitation or complexation of AS on iron oxide present in BFS (43). The CEC of filter media is another important factor to enhance the removal efficiency of arsenic. The CEC of SSF, SMF and ZMF were 4.8, 6.3 and 6.55 (meq/g), respectively. These results confirm that CEC in the zeolite is more than slag and sand. Therefore, it is clear that efficiency of zeolite media is more than other media due to high CEC.
CECZMF > CECSMF > CECSSF
Thus, the modified filters with low-cost adsorbents, Zeolite, and Blast furnace slag, had better efficiency for arsenic removal than the conventional sand filters Rany Devi et al. 2008, Shafiquzzaman et al. 2011 and Aviles et al. 2013 obtained the same results (4, 13, 24). In addition, similar results were achieved by Yu et al. 2013, Chutia et al. 2009 and Sublet et al. 2003. which used low-cost adsorbents for lead and arsenic removal (22, 44, 45).
Figure 3 shows mean removal efficiency of hardness, turbidity, and total coliforms by SSF, SMF, and ZMF. As can be seen, total hardness removal with average inlet concentration of 759 mg/L, was 21.1%, 51.9%, and 66.3% by SSF, SMF, and ZMF, respectively. This shows that ZMF can remove the higher amounts of arsenic and other pollutants compared to other studied filters (35). However, for water turbidity removal, with average influent turbidity of 30 NTU, approximately the same efficiencies of 98.8%, 98.9%, and 98.9% were achieved by SSF, SMF, and ZMF, respectively.
The world health organization (WHO) suggested 2 logs as the lowest reductions of bacteria, achieved by water treatment technologies, such as slow sand filters (16). In the present study, the total coliform of influent water was 4*106 (MPN/100 mL). The calculated bacteria reductions of the 3 investigated filter media, SSF, SMF, and ZMF were 1.97, 1.98 and 1.99 log, respectively. The conventional sand filter and modified filters by zeolite and slag had almost the same removal efficiency of coliform bacteria. Because the main reason for the removal of coliform bacteria in filters bed biological film (Schmutzdeck) formed on the surface of the filter. The biological film was present on each of the three filters.
Similar results were achieved by Baig et al. 2011 and Mahmood et al. 2011 for total coliforms removal (10, 46).
It was noted that the difference in the removal percentage of arsenic, total hardness, total and fecal coliform, and turbidity could be related to the difference in tendency of these pollutants for adsorption towards the studied filter media (4).
5.1. Conclusions
It is concluded from this study that slag and zeolite modified filters, especially zeolite, could be used for water treatment in many rural areas or small communities of developing countries that have hardness, arsenic, turbidity, and coliform problems in their water resources. Other reasons may be that these materials are low cost, and also their assembling is easy. For the promotion of these filter efficiencies in future studies, it is recommended to enhance media depth and surface.