1. Background
Some ototoxic chemicals may lead to the damage or loss of sensory cells and peripheral nerve endings of the cochlea in the inner ear (1). In industry workers, apart from occupational exposure, the hearing level can be affected by chemicals in the cigarette smoke, as smoking is a common habit among these workers.
Cigarette smoking can promote oxidative stress by generating reactive oxygen species (ROS) and reactive nitrogen species, causing damage to the cell structure and impairing cellular function. Oxidants may be formed either directly by cigarette smoke constituents or in response to cell inflammation due to smoke-related oxidative stress (2). Furthermore, increased blood viscosity and oxygen reduction due to cigarette smoking can disrupt and damage the hair cells in the cochlea (3).
The association between smoking and hearing loss has been discussed with widely diverse outcomes. Some studies have revealed that all types of direct and passive smoking are associated with conductive and sensorineural hearing loss (4-9). Some studies have also reported this association under certain conditions. Moreover, the relationship between heavy smoking and poor hearing thresholds has been reported in populations without any major noise exposure (10, 11).
In a cohort study, the number of cigarettes per day and duration of smoking were related to hearing loss, mainly at high frequencies. The harmful effect of smoking on hearing was cumulative and permanent, as high-frequency hearing loss was detected in long-term smoke exposure (11). In addition, in an animal study, nicotine receptors were detected in the hair cells, indicating that smoking might produce direct ototoxic effects on hair cell function and reduce the potential of the hearing neurotransmission organ. Hair cell damage could be a result of inadequate available oxygen for the organ of Corti and insufficient available energy for the cochlea (12). On the other hand, some studies could not detect any association (13, 14).
The controversial results regarding the relationship between smoking and hearing loss are mainly related to confounders in human studies. There are many confounders at workplace, such as exposure to ototoxic agents other than smoke, noise, and antibiotic consumption, which might be responsible for this discrepancy. Additionally, self-report information about smoking dosage and lack of knowledge about various types of smoke exposure can explain this controversy. Therefore, it is necessary to control such confounding factors through in vivo studies.
Smoking is an established risk factor for the development of arteriosclerosis and consequently ischemia (15). The most reliable monitoring tool to follow-up cochlear ischemia and reperfusion is the otoacoustic emission (OAE) test, which shows a better performance than the gross cochlear potential (15). OAE is able to differentiate neurological and auditory sensory disorders and monitor changes in the cochlear status. OAE test provides information about the middle-ear function and active biological processes within the cochlea to determine the outer hair cell (OHC) cochlear mechanism (8).
Although some animal studies have mainly focused on the effects of each ototoxic agent on hearing, there is limited evidence in animal research about the combined effects of agents in cigarette smoke, including organic solvents (styrene, benzene, and toluene), asphyxiants (carbon monoxide and hydrogen cyanide), and heavy metals (1, 16-24).
If a combination of cigarette compounds has synergistic or additive effects on hearing, as indicated in some ototoxic chemicals, development of hearing loss may be even accelerated (7). Therefore, in this study, we investigated the effect of smoking on temporary and permanent hearing loss in rats, using distortion-product OAEs (DPOAEs) as a type of OAE test.
2. Objectives
The aim of the current study was to investigate the relationship between cigarette smoking and DPOAEs in rats.
3. Methods
3.1. Experimental Groups
In this experimental study, 12 male Wistar rats (weight, 275 ± 25 g) were selected from the animal research center of Zahedan University of Medical Sciences, Zahedan, Iran and were randomly allocated into 2 groups of 6 rats, including the control group without exposure and the smoke-exposed group. In all rats, the external ear canal was examined for debris, and otoscopic examination was carried out to select rats with a normal outer ear canal and tympanic membranes.
3.2. Animal Laboratory Conditions
The rats were housed in polypropylene cages (40 × 20 × 15 cm3) 1 week before the onset of the experiment. Tap water and food were available ad libitum, except during exposure. Temperature in the animal quarter was maintained at 21 - 23°C with a relative humidity of 40% - 50%. The lights were kept on for 12 hours (07.00 am - 19.00 pm). We tried to reduce animal suffering as much as possible and decrease the number of animals needed for the experiment. All the procedures for care and use of animals were in accordance with the Declaration of Helsinki and approved by the ethics committee for experimental Medicine, Tarbiat Modares University, Tehran, Iran.
3.3. Whole-body Exposure Chamber
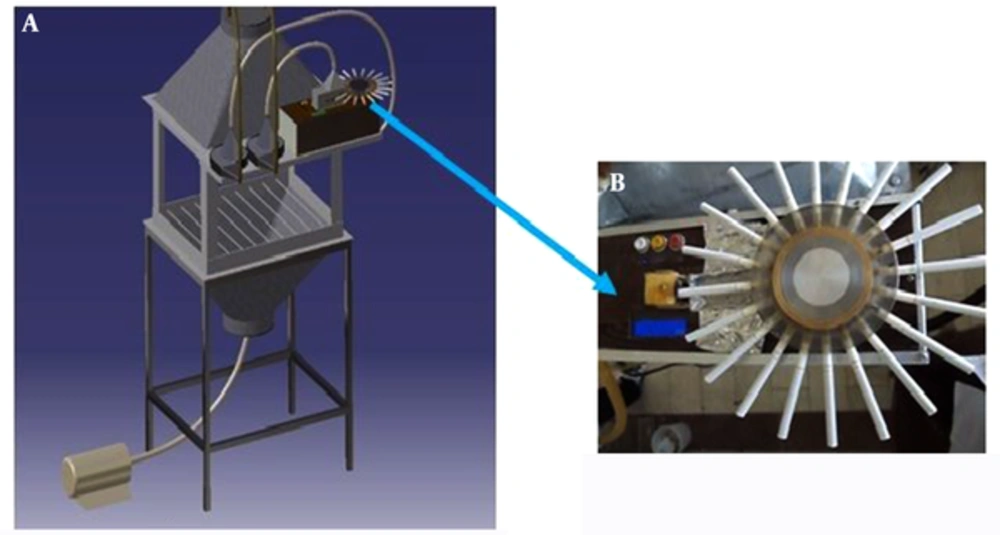
In this study, an inhalation whole-body exposure chamber was constructed. The primary design was based on the proposed considerations in previous studies, including reasonability, practicality, feasibility of animal activity, ease of maintenance, control of temperature and humidity, and maintenance of a continuous flow of fresh air (25-30). The chamber was fabricated from aluminum sheets with an outside dimension of 60 × 45 × 30 cm3 and an internal total volume of 177 L.
Two funnels (height, 30 cm) were connected to the top and bottom of the chamber with 52° and 30° slopes, respectively; this arrangement helped smoke disperse uniformly. In order to monitor the processes inside the chamber, all sides of the chamber were made of thick plate glass (Figure 1A). The chamber had enough capacity for 6 cages (dimension, 15 × 13 × 17 cm3). Open wire mesh cages were made of stainless steel with a removable lid. Each rat was placed in a cage separately, and all 6 rats were placed systematically on parallel wire shelves inside the chamber during the experiment.
The chamber was tangentially connected to a custom-made smoking machine through a short tube (diameter, 1 cm) at the top of the chamber. Two centrifugal fans (Silent Servo Blower, SCBD24Z7 model, 10 w, Japan) were used to pump either smoke or fresh air into the chamber. The air and cigarette smoke flowed into the chamber in a mild swirling motion (due to the tangential connection of tubes) and passed through a perforated plate, installed on top of the chamber inside. As a result, the air and smoke were mixed and distributed uniformly throughout the chamber.
Near the bottom of the chamber, the exhaust air was drawn axially by an exhaust system through an individual exhaust duct. Inside the chamber, a thermometer humidity device (CEM DT-625, China) was used to measure temperature and humidity, maintained at 24 - 26°C and 40% - 50%, respectively. The airflow was measured by a thermoanemometer (VT100, Kimo Instruments, UK) and controlled by adjusting the total exhaust flow (5 L/min) through the chamber to maintain a negative static pressure (Figure 1A).
3.4. Cigarette Smoke Exposure
The rats were separately placed in cages inside the chamber and were exposed to cigarette smoke for 8 hours a day during 10 consecutive days (subacute exposure). The main and side stream smoke was generated in accordance with the federal trade commission (FTC) regimen (2-second puff per minute with a volume of 35 mL and a flow of 1.05 L/min) (31). A custom-made smoking machine (Figure 1B), burning 20 cigarettes (9 ± 1 mg of tar and 0.8 ± 0.1 mg of nicotine per cigarette) was used during 8 hours a day.
The 8-hour exposure consisted of 2 repetitions of cigarette smoking cycles. In the first cycle, the rats were passively exposed to the smoke of 1 cigarette for 8 minutes (2 seconds of main stream and 58 seconds of side stream smoke), including 8 puffs per cigarette. In the second cycle, fresh air was allowed to be introduced instead of smoke for 17 minutes; these 2 cycles were repeated to burn 20 cigarettes.
3.5. Air Sampling from the Chamber Atmosphere
The test atmosphere was sampled, using a standard 0.9-cm nominal pipe, which was inserted in the chamber through 4 holes (diameter, 1.2 cm), drilled, and then tapped on every side. Real-time sampling of the total suspended particulates (TSPs) was performed through a 4-cm hole in front of the chamber door. All the samples were obtained in the breathing zone of animals. Concentrations of 42.7 ± 5.7 mg/m3 for TSP and 300 ppm for carbon monoxide (CO) were monitored and maintained in the chamber with a Microdust Pro Instrument (Microdust ProTM, Real-Time Aerosol Monitor Kit, Casella Cel Inc.) and a combustion analyzer (MRU Delta 65-s, Germany), respectively.
3.6. Electrophysiological Test
The rats were anesthetized with a mixture of ketamine (68 mg/kg) and xylazine (8 mg/kg) via intraperitoneal injection. The DPOAEs were measured inside a small sound-attenuated chamber, lined with acoustic foam tiles with sound pressure not exceeding 42 dB. All DPOAE tests were performed by a researcher, and the DPOAE measurements were repeated 3 times for each rat; the average value was considered as the DPOAE level.
A standard commercial cochlear emission analyzer (ECL 14091 apparatus, Labat’s EchoLab Ltd, Italy) was used for the DPOAE test. The device was calibrated at the official branch of the company in Iran before starting the experiments. Calibration was also achieved automatically prior to each test for every ear. A frequency range of 4620 - 9960 kHz was considered for the bandwidth of DPOAE responses (2f1-f2, referenced to f2) in order to avoid the influence of standing waves in the external meatus; also, 12 points were sampled per octave (32).
The primary f2/f1 frequency ratio was assumed to be 1:21. A nonsymmetrical DPOAE protocol was used to evoke responses, based on unequal primary tone stimulus intensities, i.e., L1 s L2; in fact, cochlear dysfunction can be more precisely evaluated with such stimulus intensities. The protocol was defined as follows: high level, L1 = 60 dB SPL and L2 = 50 dB SPL. All measurements ≥ 3 dB regarding signal-to-noise ratio (SNR) were used for the analysis. A heating blanket was used to keep the body temperature constant between 37.5 and 38.58°C. DPOAE test was performed at baseline before the intervention and 1, 7, and 21 days after smoke exposure on the left ear of all rats.
3.7. Statistical Analysis
Shapiro-Wilk test was used to evaluate the normal distribution of the data. Repeated measures ANOVA and 2-sample independent t test were performed for data analysis, using SPSS version 18. The significance level was set at 0.05.
4. Results
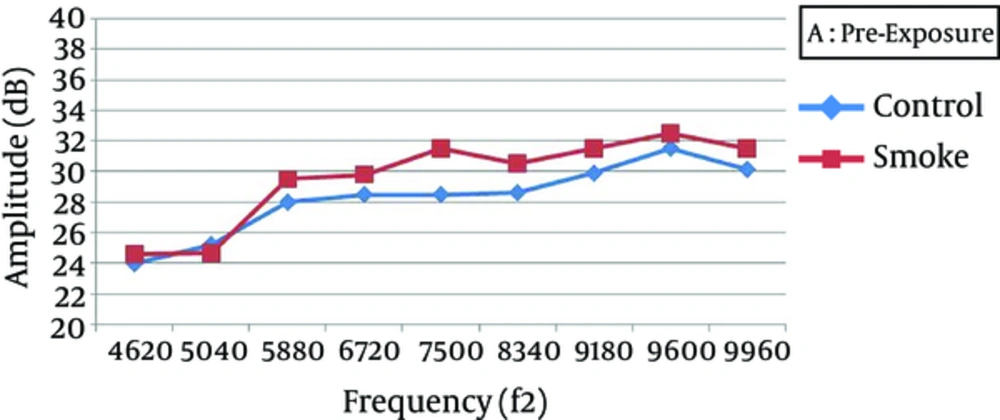
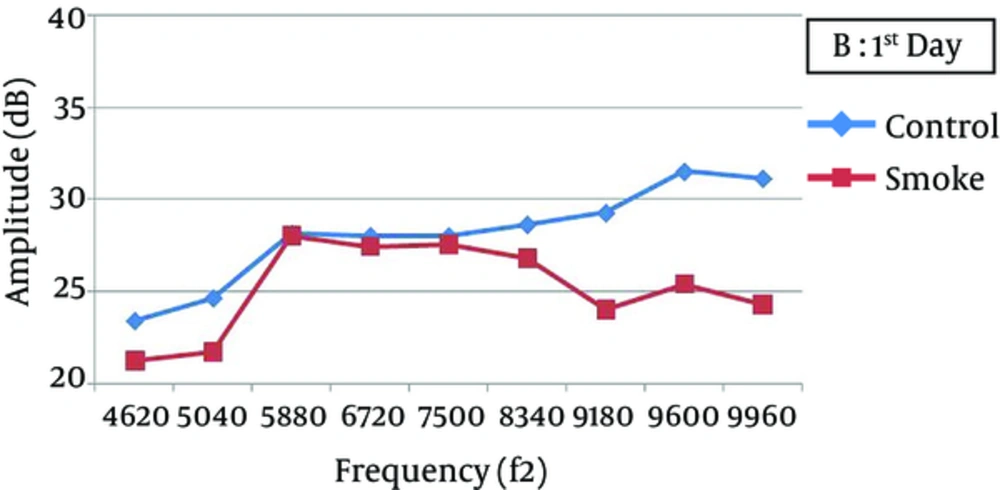
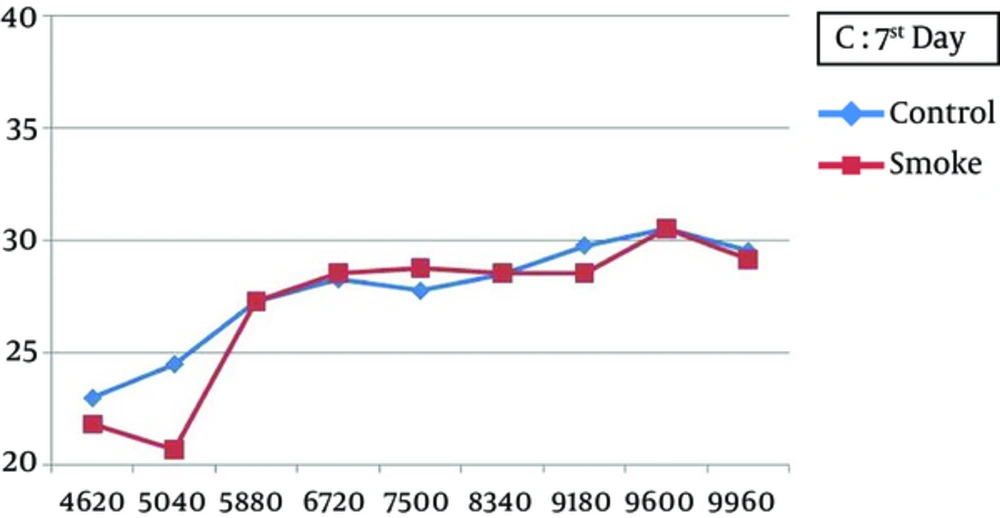
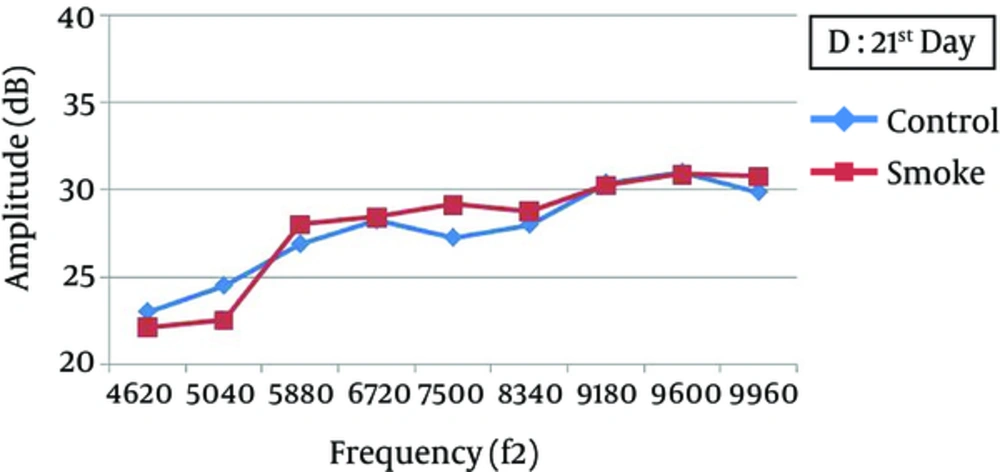
At all frequencies, the baseline amplitudes were not significantly different between the smoke-exposed and control groups (Figure 2). In the control group, the DPOAE amplitudes did not significantly vary after 1, 7, and 21 days, while there was a significant difference between the baseline and 1-day post-exposure measurements (P < 0.05), with a greater attenuation at higher frequencies (7500 - 9960 Hz) (Figure 3). The amplitude obviously recovered in the exposed animals from day 1 to day 7 post-exposure, and further improvement was observed until the end of the experiment (Figures 4 and 5).
One day after the intervention, the average DPOAE amplitudes in the smoke-exposed animals were attenuated by nearly 1.5 - 3.4 dB at lower frequencies (4620 - 6720 Hz) and 3.75 - 7.50 dB at higher frequencies (7500 - 9960 Hz), relative to the baseline (temporary hearing changes). Except for 4620 Hz frequency, the reduction was significantly different from the control animals. However, the average DPOAE amplitude gradually recovered after 21 days of exposure and approached the baseline. Therefore, DPOAE amplitude reduction, relative to the baseline, was not significantly different between the smoke-exposed and control groups after 7 and 21 days (Table 1). Also, 21 days after exposure, permanent amplitudes were not significantly different between the control and smoke-exposed groups at different frequencies (P > 0.05).
| Variables | Baseline-Day 1 (Temporary Hearing Changes) | Baseline-Day 7 | Baseline-Day 21 (Permanent Hearing Changes) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Control | Smoke exposure | P value | Control | Smoke exposure | P value | Control | Smoke exposure | P value |
| 4620 | 0.62 (2.21) | 3.40 (1.34) | 0.082 | 1.00 (0.50) | 2.80 (3.27) | 0.314 | 1.00 (1.15) | 2.50 (1.50) | 0.145 |
| 5040 | 0.62 (0.75) | 3.00 (1.00) | 0.015 | 0.75 (1.50) | 3.33 (2.89) | 0.179 | 0.75 (0.50) | 2.17 (1.04) | 0.059 |
| 5880 | -0.12 (1.03) | 1.50 (0.58) | 0.043 | 0.75 (0.87) | 2.25 (0.87) | 0.051 | 1.12 (2.32) | 1.50 (2.08) | 0.818 |
| 6720 | 0.50 (1.00) | 2.40 (0.55) | 0.008 | 0.25 (0.5) | 1.30 (2.33) | 0.378 | 0.25 (1.50) | 1.40 (0.89) | 0.194 |
| 7500 | 0.50 (1.00) | 4.00 (0.82) | 0.002 | 0.75 (1.66) | 2.75 (1.71) | 0.144 | 1.25 (1.50) | 2.38 (1.80) | 0.374 |
| 8340 | 0.00 (2.04) | 3.75 (2.22) | 0.047 | 0.12 (2.46) | 2.00 (3.46) | 0.412 | 0.62 (1.11) | 1.75 (1.55) | 0.283 |
| 9180 | 0.62 (3.04) | 7.50 (2.52) | 0.013 | 0.12 (1.65) | 3.00 (4.69) | 0.292 | -0.50 (2.27) | 1.25 (3.40) | 0.425 |
| 9600 | 0.00 (1.15) | 7.12 (2.53) | < 0.001 | 1.00 (0.82) | 2.00 (0.82) | 0.595 | 0.50 (1.29) | 1.38 (0.75) | 0.402 |
| 9960 | -1.00 (2.12) | 7.25 (2.06) | 0.001 | 0.62 (2.21) | 2.38 (3.14) | 0.398 | 0.25 (2.18) | 0.75 (4.79) | 0.855 |
The Mean (Standard Deviation) DPOAE Amplitude Differences Between the Baseline And Postexposure Measurements in the Control and Smoke-Exposed Groups
The increasing trend of hearing recovery from day 1 to day 7 post-exposure was not constant at all frequencies. Based on the findings, at lower frequencies (4620 - 8340 Hz), the amplitudes in the smoke-exposed group were not significantly different from the controls, while it was considerably larger at higher frequencies (9180 - 9960 Hz) (P < 0.05). Also, the DPOAE levels after recovery were not significantly different between the smoke-exposed and control animals at 7 and 21 days after the intervention (Table 2).
| Variables | Day 1 - Day 7 (Initial Recovery) | Day 7 - Day 21 (Subsequent Recovery) | ||||
|---|---|---|---|---|---|---|
| Frequency | Control | Smoke exposure | P value | Control | Smoke exposure | P value |
| 4620 | -0.38 (2.21) | 0.60 (2.97) | 0.603 | 0.00 (1.15) | 0.30 (2.33) | 0.822 |
| 5040 | -0.12 (1.03) | -0.33 (2.08) | 0.866 | 0.00 (1.15) | 1.17 (1.89) | 0.354 |
| 5880 | -0.88 (0.75) | -0.75 (0.29) | 0.766 | -0.38 (2.14) | 0.75 (1.44) | 0.416 |
| 6720 | 0.25 (1.50) | 1.10 (2.20) | 0.532 | 0.00 (1.82) | -0.10 (1.75) | 0.936 |
| 7500 | -0.25 (1.85) | 1.25 (2.06) | 0.320 | -0.50 (1.68) | 0.38 (2.14) | 0.544 |
| 8340 | -0.12 (2.29) | 1.75 (2.06) | 0.269 | -0.50 (1.91) | 0.25 (3.71) | 0.732 |
| 9180 | 0.50 (1.91) | 4.50 (1.91) | 0.082 | 0.62 (2.84) | 1.75 (2.06) | 0.555 |
| 9600 | -1.00 (1.41) | 5.12 (2.39) | 0.031 | 0.50 (0.58) | 0.62 (1.11) | 0.894 |
| 9960 | -1.62 (3.57) | 4.88 (2.10) | 0.015 | 0.38 (0.75) | 1.62 (1.25) | 0.247 |
The Mean (Standard Deviation) DPOAE Amplitude Differences in Postexposure Measurements in the Control and Smoke-Exposed Groups
5. Discussion
After 10 days of exposure to smoke, a significant attenuation in the emission amplitude was observed at 1 day after exposure. The amplitude reduction was more pronounced at higher frequencies; this reduction was mostly recovered after 7 and 21 days. In a previous study, reduction in DPOAE levels, without concomitant changes in the noise floors, was different among smokers and nonsmokers (33), which is in agreement with the results of the current study. Also, further dose-dependent cigarette smoke deterioration was detected at higher frequencies. Therefore, smoking increases the vulnerability of the most basal portion of the cochlea, where higher sound frequencies are transduced (5, 11, 12). It has been also shown that CO can mostly affect hearing loss at high frequencies, while long exposure to CO may affect low frequencies, as well (34).
The effect of smoke on the cochlea could be explained by possible pathophysiological mechanisms, i.e., chronic ischemia due to arteriosclerosis, elevated plasma viscosity, effect of chronic CO exposure, and direct activity of nicotine. The greater effect of CO compared to nicotine on temporary threshold shifts has been reported in the literature (35). Overall, smokers are at a higher risk of arteriosclerosis, as the number of pack years of smoking increases; also, elevated plasma levels have been reported in smokers (36).
Conversion of oxyhemoglobin to carboxyhemoglobin due to CO exposure can lead to hypoxia (37). Depletion of cellular energy stores, following prolonged hypoxia or hypoxia–ischemia, leads to neuronal and glial depolarization and release of excitatory amino acids into the extracellular space. Energy-dependent reuptake mechanisms become compromised, allowing glutamate to accumulate to excitotoxic levels. Also, overactivation of N-methyl-D-aspartate (NMDA) receptors increases the intracellular calcium levels and initiates cellular processes, culminating in cell death (38, 39).
Additionally, as DPOAEs emanate from OHCs, which are enormously sensitive to changes in oxygen and blood supply, they can be influenced by anoxic insults. Several clinical studies have reported the association between reduced emission amplitudes and anoxic insults (33). The direct action of nicotine is also possible, since nicotine receptors are found on the OHCs of the cochlea (40). The cochlear artery, which ends in high-frequency regions, is prone to the effects of atherosclerotic changes; this finding has been also reported in smokers (33). Overall, cigarette smoking increases oxidative stress, which generates ROS either directly or through activation of inflammatory cells (2, 41).
In this study, DPOAE amplitudes started recovering after 1 day of exposure to smoke. They increased until day 7 and approached the preexposure level after 21 days of the intervention. In consistence with the present study, evidence suggests that acute CO exposure generally produces changes in the audiogram, which can be recovered during several months (42). Since there are limited animal studies on cigarette smoking, the temporary effects of smoke on hearing and histological/physiological changes are not clear. However, in the present study, this level of subacute smoke exposure for 10 consecutive days resulted in temporary and reversible biological and physiological changes; also, the endogenous defense system of the cochlea was able to recover.
The mentioned findings have been confirmed in a review article on 37 animals. Changes were observed in the glutathione level and oxidative stress markers in the first 6 hours after acute cigarette smoke exposure, while these parameters returned to the normal range within 24 hours, suggesting the protective mechanism of cells against oxidative stress from smoke (2). Additionally, this recovery might be due to the exertion of free radical products after acute smoke exposure, as levels of both free and esterified F2-isoprostanes (as lipid peroxidation products) become significantly lower than the plasma levels, measured during smoking after 2 weeks of abstinence (43).
In the current research, the histological effects on the cochlea were not investigated, while in another study, nicotine injection for 1 month in guinea pigs resulted in the damage of stereocilia, including disorganization, bent and limp (or complete loss), and expansion of the surrounding supporting cells (44). Evidence suggests that buckling of pillar bodies temporarily uncouples the OHC stereocilia from the tectorial membrane; also, hair cell stimulation is attenuated by this uncoupling (45). Therefore, the degenerated number of hair cells may be larger when more TTSs are sustained by the cochlea. Irreversible hearing loss is expected if animals are exposed to cigarette smoking with higher concentrations of TSP and CO.
Since workers are exposed to cigarette smoke and different ototoxic agents at workplace for a long time, it is necessary to investigate the chronic or subchronic effects of smoke on permanent hearing damage through designing more in vivo studies. This study could not investigate hearing changes at frequencies higher than 10,000 Hz due to instrumental limitations. As more hearing attenuation was detected at higher frequencies after 1 day of exposure, use of instruments with a greater broad frequency range is recommended to obtain more accurate results about the effects of cigarette smoke on hearing loss.