1. Context
Cancer remains a formidable challenge in healthcare, demanding innovative approaches to improve patient outcomes (1). In recent years, artificial intelligence (AI) has emerged as a transformative technology with the potential to revolutionize cancer research and treatment (2). By harnessing machine learning algorithms, AI offers opportunities to enhance diagnostic accuracy, personalize treatment, optimize drug discovery, and improve patient management. This systematic review aims to explore the potential role of AI in cancer therapeutics by examining the current state of the field, recent advancements, and future directions.
The landscape of cancer therapeutics presents clinicians and researchers with significant challenges. Traditional approaches, such as surgery, chemotherapy, and radiation therapy, have improved patient outcomes; however, limitations remain, namely toxicity, suboptimal response rates, and drug resistance (3-6). Therefore, novel therapeutic strategies are urgently needed to address these challenges and improve patient responsiveness and survival.
Artificial intelligence, particularly machine learning algorithms, has shown great promise in cancer research (7). By analyzing vast amounts of patient and clinical data, including genomic profiles, medical images, and electronic health records, AI-based algorithms can identify signatures, detect anomalies, and generate predictive models (8-10). This capability generally enables AI to aid in cancer diagnosis, prognosis, and treatment selection, resulting in more precise and personalized therapeutic interventions.
The era of precision medicine emphasizes the need for tailored treatment approaches that consider individual patient characteristics. Artificial intelligence can make a significant contribution to this paradigm shift by integrating multi-omics data and clinical parameters to predict treatment responses and guide therapeutic decision-making (11). Artificial intelligence algorithms can identify molecular patterns associated with drug sensitivity or resistance, enabling the selection of targeted therapies with higher efficacy and lower toxicity.
In addition to diagnosis and treatment, AI can play a crucial role in patient management and monitoring. Remote patient monitoring systems, powered by AI algorithms, continuously analyze patient-generated data to detect early signs of treatment toxicity, disease progression, or treatment response (12). AI-driven decision support systems aid clinicians in treatment planning, dose adjustment, and identifying potential adverse events, ultimately improving patient care and safety (13).
Research results show that AI significantly improves the accuracy and sensitivity of diagnoses, especially in the early detection of cancers such as lung, breast, and colon cancer (14, 15). In the review study by Chen et al., the application of AI in assisting with the early diagnosis of cancer, classification and grading of the disease, predicting patient outcomes and treatment responses, personalized treatment, the discovery of new anti-cancer drugs, and clinical trials was also mentioned (16). In addition, studies show that the use of some features of AI programs allows for accurate prediction and diagnosis of diseases, especially in non-communicable chronic diseases (14).
The field of AI in cancer therapeutics is rapidly evolving. Advancements in machine learning, natural language processing, and explainable AI will enhance the accuracy, interpretability, and clinical utility of AI algorithms. Establishing robust guidelines and frameworks for the responsible development and deployment of AI technologies in cancer care is crucial to ensuring patient safety, maintaining trust, and upholding ethical standards.
2. Methods
2.1. Study Design
The present study is a literature review, analyzing research papers on AI technology utilization and its implications for cancer treatment in clinical settings. This study was conducted following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline (17).
2.2. Search Strategy
The following databases were searched to retrieve all literature with meaningful and appropriate data regarding the research topic: PubMed, Scopus, and Web of Science, from October to 30 December 2023. The search terms included the following keywords and medical subject headings (MeSH) terms with various combinations. The search strategy for the PubMed database is presented below:
("artificial intelligence"[Title/Abstract] OR "artificial intelligence"[MeSH] OR "Computational Intelligence"[Title/Abstract] OR "Machine Intelligence"[Title/Abstract] OR "Computer Reasoning"[Title/Abstract] OR "machine learning"[Title/Abstract]) AND ("Tumor"[Title/Abstract] OR "Neoplasm"[Title/Abstract] OR "Neoplasms"[MeSH] OR "Cancer"[Title/Abstract] OR "malignan*"[Title/Abstract]) AND ("therapy*"[Title/Abstract] OR "Therapeutics"[MeSH] OR "treatment"[Title/Abstract] OR "disease management"[Title/Abstract] OR "Chemotherapy"[Title/Abstract] OR "drug therap*"[Title/Abstract] OR "Pharmacotherapy"[Title/Abstract] OR "Radio therapy"[Title/Abstract] OR "Immunotherapy"[Title/Abstract]).
2.3. Study Selection
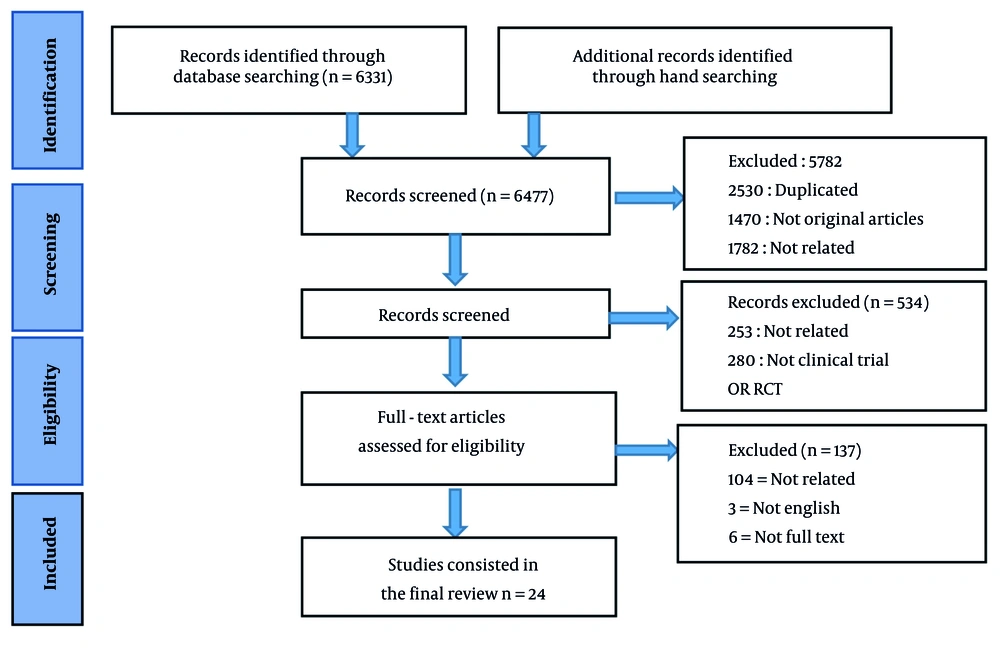
After removing the duplicates, based on the inclusion criteria, the titles and abstracts of the articles were reviewed by two independent reviewers, and if there was any disagreement, the opinion of a third reviewer was used. In addition, the full texts of the remaining publications were obtained and screened to determine the eligibility of each study. The included studies investigated the contribution of AI to the improvement of cancer therapeutics or cancer treatment strategies in clinical settings or clinical outcomes of cancer patients. The exclusion criteria were as follows: (1) Non-original studies, including reviews, guidelines, conference presentations, or comments; (2) studies that did not undergo peer review; (3) studies published in languages other than English; and (4) studies without an available full text. The details of the selection process of the articles based on the PRISMA statement are presented in Figure 1.
2.4. Data Extraction
Two independent reviewers screened the included articles to obtain data such as the first author, publication year, country of origin, patient characteristics, treatment procedure and strategy, applied AI, clinical outcome or treatment response, and any disagreements were resolved with the third reviewer. For duplicate papers or research studies presenting the same cohort data, only the most recently published article was used.
2.5. Quality Assessment
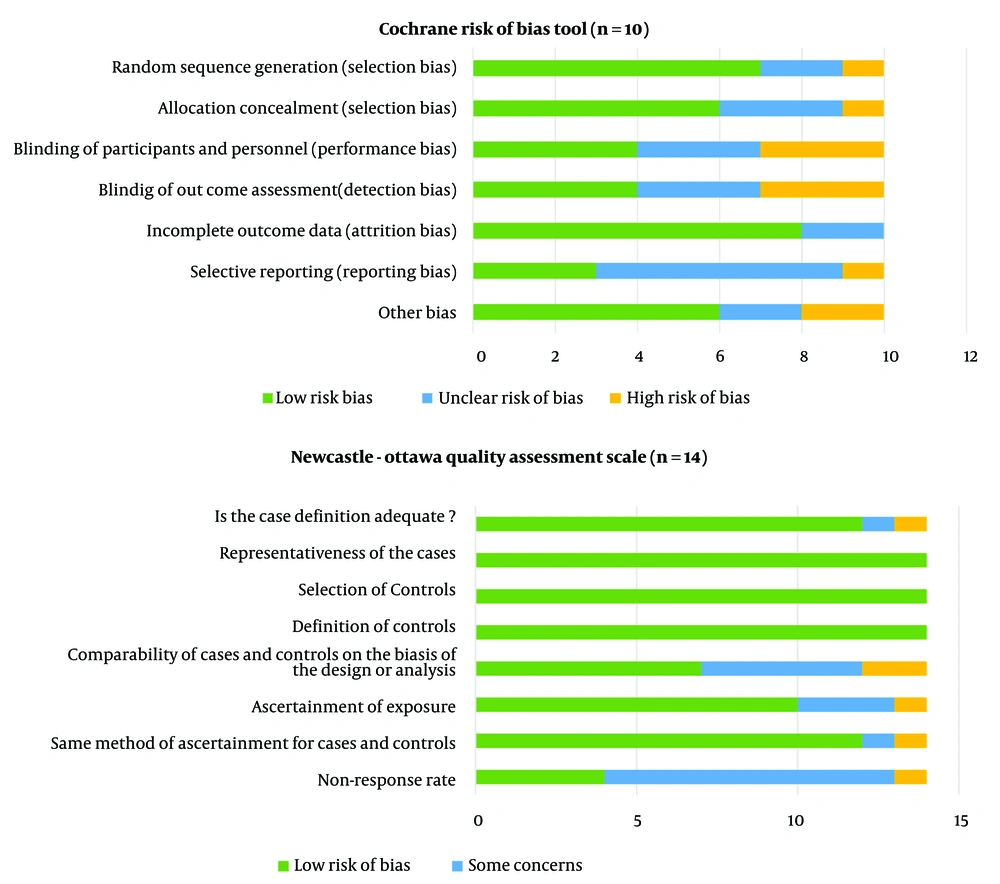
The quality of the included studies was assessed independently by two reviewers (AA and ASH), and any disagreements were resolved through further review and the opinion of a third reviewer (AK). The quality appraisal of the enrolled studies was conducted using the Newcastle–Ottawa Scale (NOS) with the standard 9-point scale, which includes three criteria: Selection, comparability, and exposure, and the Cochrane Risk of Bias tool, which includes five criteria: Selection, performance, detection, attrition, and reporting bias (18).
3. Results
3.1. Search Results
The systematic literature search identified 24 relevant studies that met the inclusion criteria (Figure 1). Ten of the included articles (41.6%) were designed as clinical trials, and the methodology of 14 articles (58.4%) was randomized clinical trials. The studies were categorized based on their primary focus on AI applications in cancer therapeutics, including drug discovery, precision medicine, image analysis, and treatment optimization in clinical settings. Among the 24 studies included in this systematic review, 11 (45.8%) focused on patient response to cancer treatment, 8 (33.3%) discussed precision medicine, and 5 (20.9%) described AI applications for predicting cancer treatment adverse events.
3.2. Quality Assessment Result
The results of the quality assessment of the 24 articles included in the present study, using the NOS and the Cochrane risk of bias tool, showed that most of the studies reviewed had a high level of quality and exhibited a low risk of bias. Details are shown in Figure 2.
3.3. Treatment Response Prediction
Over the past few decades, remarkable progress has been made in the development of novel cancer treatment strategies, leading to improved clinical outcomes for many patients. Conventional approaches to cancer treatment, such as surgery, chemotherapy, and radiation therapy, have been coupled with targeted therapies and immunotherapies, which have shown great promise in treating various types of cancer (19). However, despite these advancements, there is still a need for more personalized and precise treatment approaches to improve patient outcomes and reduce the burden of cancer (20).
One major area where the development of cancer treatment strategies has been particularly challenging is in predicting treatment responses and clinical outcomes. Currently, the recommendation of treatment options for cancer patients is mainly based on the type and stage of the cancer, as well as the patient's characteristics, overall health, and medical history (21). However, these factors may not accurately predict patient response to a particular treatment. Therefore, there is growing interest in the emergence of AI-based tools that can analyze a patient's unique genetic and molecular profile to predict their response to different treatment options and improve clinical outcomes. Table 1 presents a summary of the clinical trial studies that have utilized AI-based approaches for the prediction of cancer treatment responses.
| Authors (Year) | AI Application | Patient Characteristics | Study Methodology | Sample Size | AI Algorithms Used | Key Findings |
|---|---|---|---|---|---|---|
| Wei et al. (2023) (22) | Peripheral blood immune cell markers | Cancer patients who received ICI therapy | Dynamic monitoring of peripheral blood immune cell markers | 160 | Lasso, ASR, and Cox models | Development of AI-based non-invasive, specific, and sensitive monitoring model |
| Chen et al. (2022) (23) | Pretreatment radiomics features | Non-small cell lung cancer patients who received definitive concurrent chemoradiotherapy | Identification of pre-treatment computed tomography-based radiomics features | 298 | Integrated feature selection and model training approach | Identification of 9 features for long-term predicting patient’s survival after treatment |
| Ali et al. (2016) (24) | Digital pathology slides | Breast cancer patients who received neoadjuvant chemotherapy | Evaluation of computational metrics from tissue pathology | 768 | Univariate and multivariate logistic regression | I dentification of lymphocyte density as an independent predictor |
| George et al. (2022) (25) | MRI radiometric | Glioblastoma patients who received immunotherapy | Progression-free survival and overall survival of patients with MRI-based machine learning | 113 | Random survival forest algorithm | Development of MRI radiometric-based AI model to predict patient survival |
| Pfob et al. (2022) (26) | Vacuum-assisted biopsy | Breast cancer patients who received NST | Detection of residual cancers using patient, imaging, tumor, and vacuum-assisted biopsy variables | 318 | Extreme Gradient Boosting Tree algorithm | Development of an intelligent vacuum-assisted biopsy model to identify NST responders |
| González-Garcia, I. et al. (2020) (27) | Tumor size data and patients’ demographic and clinical data | Head and neck cancer patients who received immunotherapy | Prediction of overall response and survival | 482 | Nonlinear mixed-effects modeling and a machine learning classification algorithm | Development of computational frame work to predict overall response and survival |
| Terranova et al. (2021) (28) | Patients’ characteristics and laboratory biomarkers | Gastric cancer or gastroesophageal junction cancer who received chemotherapy | Prediction of long-term overall survival | 805 | Random forests, SIDEScreen, and variable-importance assessments | Identification factors associated with overall survival including age, gamma-glutamyl transferase concentration, absence of peritoneal carcinomatosis; neutrophil-lymphocyte ratio, lactate dehydrogenase, or C-reactive protein |
| Wilbaux et al. (2022) (29) | Patients’ characteristics | Hepatocellular carcinoma patients who received Roblitinib | Prediction of tumor growth inhibition profile | 127 | Random forest, neural net, and support vector machine | Development of a machine learning model for the prediction of pharmacokinetic based on patient’s baseline characteristics |
| Wang et al. (2020) (30) | Cytokine data | Cancer patients who received nivolumb monnotherapy | Prediction of overall survival | 468 | Random forest (Boruta) algorithm | Prediction of overall survival using 16 immunomodulatory cytokines signature |
| Shipp et al. (2002) (31) | Expression levels of 6817 genes in tumor biopsy | Diffuse large B-cell lymphoma patients who received CHOP-based chemotherapy | Prediction of clinical outcomes by supervised learning method | 77 | Weighted-voting algorithm and cross-validation testing | Classification of two patient categories with significantly different 5-year overall survival rate |
| Deng et al. (2022) (32) | Clinicopathological characteristics and radiomics features of the tumor lesion | Hepatocellular carcinoma patients who underwent radical hepatectomy | Prediction of overall survival | 150 | LASSO algorithm | Prediction of patients’ overall survival with alpha-fetoprotein, neutrophil-to-lymphocyte ratio, and radiomics signature |
Summary of the Studies on the Application of Artificial Intelligence for Treatment Response Prediction
The integration of AI-based tools into cancer treatment strategies has the potential to renew the clinical settings for cancer care. By leveraging the power of machine learning algorithms and big data analytics, we can analyze complex demographic, biological, and clinical data to identify signatures and predict treatment responses with a high degree of accuracy. This could lead to more personalized and precise treatment plans for cancer patients, eventually improving their overall survival rate and quality of life. Recently, Wei et al. (22) developed a prediction model based on peripheral blood immune cell markers to estimate patient responses to cancer immunotherapy. Similarly, George et al. (25) developed a magnetic resonance imaging (MRI) radiometric-based machine learning model to predict glioblastoma patient survival following immunotherapy. Several other AI-based models have been developed for the estimation of cancer treatment responses for novel (22, 25, 27) and conventional therapeutics (23, 24, 28). As the field of AI continues to advance, researchers and clinicians must work together to develop and validate new guidelines and frameworks for use in clinical practice, ultimately improving the effectiveness of cancer treatment approaches.
3.4. Treatment Adverse Events Prediction
Beyond the prediction of treatment response and clinical outcomes, there is an urgent need for the development of AI methods to predict potential adverse events related to cancer therapeutics, such as chemotherapy toxicity and vomiting (33, 34). Chemotherapy, while effective in eliminating cancer cells, can also cause considerable side effects that vary from patient to patient. These adverse events can have a profound impact on a patient's quality of life and may even lead to treatment interruptions or discontinuations (35). These side effects often occur during and continue after the duration of treatment or emerge among the survivors long after the course of therapy (36).
By utilizing AI-based tools to analyze patient data, including genetic and molecular profiles, as well as clinical and demographic information, clinicians can better predict and manage potential adverse events, leading to more personalized and tailored treatment regimens for cancer patients.
The development of AI methods for predicting treatment-related adverse events is particularly important in the context of precision medicine, which seeks to tailor medical treatment to the individual characteristics of each patient. Table 2 presents the data regarding the clinical application of machine learning models for predicting and determining potential side effects related to cancer therapeutics. These models can identify and classify patients who may be at higher risk for specific treatment-related toxicities; therefore, proactively adjust their treatment plans to minimize these risk factors. This personalized approach to cancer care has the potential to improve patient outcomes and reduce the burden of treatment-related side effects, ultimately leading to a better overall quality of life for cancer patients.
| Authors (Year) | AI Application | Patient Characteristics | Study Methodology | Sample Size | AI Algorithms Used | Key Findings |
|---|---|---|---|---|---|---|
| Zheng et al. (2022) (37) | 46 clinical and drug-related variables | Esophageal cancer patients who received chemotherapy | Predicting chemotherapy-related adverse events | 1446 | Random forest | Prediction of myelosuppression incidence to provide preventative measurements |
| Mei et al. (2022) (34) | Vomiting, psychological state, quality of life, and cancer biomarkers | Lung cancer patients who received chemotherapy | Development of comfort care to reduce chemotherapy-related adverse events | 118 | Diffusion-weighted imaging under the weighted nuclear norm minimization noise reduction algorithm | AI-based comfort care can significantly ameliorate vomiting response after chemotherapy |
| Ou et al. (2022) (38) | Pre-treatment peripheral blood test | Cervical cancer patients who underwent radical hysterectomy | Development of predictive algorithm for surgical-related adverse events | 1260 | Gradient Boosting Machine, Support Vector Machine with Gaussian kernel, Random Forest, Conditional Random Forest, Naive Bayes, and Elastic Net | Prediction of pathologic risk factors in cervical cancer patients prior to surgical intervention |
| Dercle et al. (2022) (39) | CT images | Melanoma patients who received immunotherapy | Prediction of overall survival and estimation of treatment benefits | 575 | Random forest | Identification of a signature from CT image features to estimate overall survival for 6 months and predict the potential immunotherapy risk factors |
| Bedon et al. (2021) (33) | Clinical, blood biochemistry, and genotype data | Metastatic colorectal cancer patients who received chemotherapy | Pre-treatment prediction of chemotherapy toxicity | 45 | Random forest | Identification of hemoglobin, serum glutamic oxaloacetic transaminase, and albumin as predictive factors related to chemotherapy toxicity |
Summary of the Studies on the Application of Artificial Intelligence for Cancer Treatment Adverse Events
3.5. Precision Medicine
The integration of machine learning methods for predicting treatment response and therapeutics-related adverse events can facilitate the promotion of comfort care and optimize resource allocation in healthcare settings (40, 41). By identifying patients who are at higher risk for specific toxicities, clinicians can prioritize interventions and supportive care measures. This, in principle, leads to the improvement of the efficiency and effectiveness of cancer treatment. This targeted approach to supportive care has the potential to reduce healthcare costs and improve patient satisfaction, ultimately resulting in higher overall response and survival rates for cancer patients (42).
Some clinical trial studies have evaluated the utilization of AI-based methods for the improvement of precision medicine and the development of tailored treatment options. As such, Xu et al. (43) developed a classification model to cluster cancer patients based on pre-treatment symptom severity and thus recommend a distinct treatment regimen for each cluster. Sove et al. (44) presented a computational approach to group hepatocellular carcinoma patients based on their underlying pathophysiology. These methods can also improve the decision tree and help clinicians make timely and efficient decisions (45). Table 3 presents a data summary of the clinical studies that used AI-based methods to promote and improve precision medicine.
| Authors (Year) | AI Application | Patient Characteristics | Study Methodology | Sample Size | AI Algorithms Used | Key Findings |
|---|---|---|---|---|---|---|
| Xu et al. (2023) (43) | Pre-treatment symptoms | Older adults with advanced cancer who will receive a new treatment | Unsupervised machine learning to cluster patients’ symptom severity | 706 | K-means with Euclidean distance algorithm | Clustering patients with different symptom severity for administration of distinct treatment regimens |
| Sove et al. (2022) (44) | Laboratory biomarkers | Hepatocellular carcinoma patients who will receive nivolumab and ipilimumab | Conducting virtual clinical trial | 5000 | Markov Chain Monte Carlo optimization algorithm | Patient selection conducted based on the underlying pathophysiology |
| Sasak et al. (2021) (45) | Patient characteristics, and cytogenetic and molecular response | Chronic myeloid leukemia patients who will receive tyrosine kinase inhibitors | Optimization treatment approach | 504 | Ensemble learning with XGBoost package and hyperparameter optimization | Improvement of decision tree method for optimal treatment suggestion |
| Nicolae et al. (2020) (46) | Dosimetry recommendation | Prostate cancer patients who will receive I-125 low-dose-rate monotherapy | Evaluation of AI-based treatment recommendation and conventional manual recommendation | 41 | Adaptation of prostate implant planning algorithm | AI produced timely and efficient recommendation that was a non-inferior postoperative dosimetry to that of expert |
| Kaidar-Person et al. (2023) (47) | Clinical data report forms | Breast cancer patients who will receive a new treatment | Evaluation of AI-based treatment recommendation and standard conventional recommendation | 515 | Multinomial regression models | Pre-treatment AI evaluation can result in higher satisfaction, well-being, and better psychosocial status |
| Li et al. (2023) (48) | Patient and tumor characteristics, and treatment details | Hepatocellular carcinoma patients who will receive a new treatment | Presentation of surgery and chemotherapy treatment options | 1136 | Cox proportional hazards mode, neural network multitask logistic regression, DeepSurv, and random survival forest | Substantial improvement of chemotherapy recommendation therapies |
| Liu et al. (2022) (49) | The European Organization for Research and Treatment of Cancer quality of life questionnaire | Thyroid cancer patients who underwent thyroidectomy | Prediction of quality-of-life following thyroidectomy | 286 | Random forest | Optimization of health care by accurately predicting quality of life for 3 months |
| Al‑Hilli et al. (2023) (50) | Survey evaluating their knowledge of breast cancer and evaluating their satisfaction with their decision | Patients with breast cancer who will undergo genetic testing | Evaluating Chatbot counseling with in-person testing | 37 | Not Available | Comparable satisfaction and comprehension in patients undergoing pre-test genetic counseling with Chat bot to in-person testing |
Summary of the Studies on the Application of Artificial Intelligence for Precision Medicine
3.6. Emerging Framework
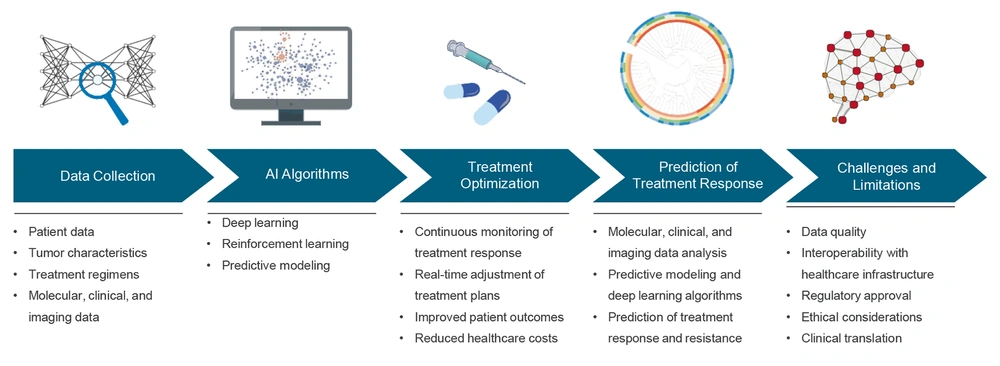
The emerging framework of AI integration with clinical settings holds great promise for improving treatment response, reducing treatment adverse events, and personalizing treatment strategies for cancer patients (Figure 3). One key aspect of this framework is data collection, which involves gathering and analyzing a wide range of patient data (51-53). This comprehensive data collection will allow AI algorithms to identify patterns, signatures, and correlations that can be used to predict treatment responses and potential risk factors.
Emerging framework for the use of artificial intelligence (AI) in cancer treatment – the figure illustrates the components of the emerging framework for the use of AI in cancer treatment, including data collection, AI algorithms, and treatment optimization, deep response, and challenges and limitations.
The next step in this framework involves the utilization of AI algorithms for treatment optimization (54). Using machine learning and big data analysis, AI algorithms can analyze complex patient data to identify the most effective and tolerable treatment strategies for cancer patients.
Furthermore, the integration of AI with clinical settings allows for the prediction of treatment response, which is critical for optimizing treatment plans according to the unique characteristics of each individual (55). Therefore, clinicians can better foresee how a patient might respond to a distinct therapeutic.
However, this framework also comes with its own set of challenges and limitations. One of the primary challenges is the requirement for robust and high-quality data to train AI algorithms effectively (56). Additionally, there are ethical and regulatory considerations that are yet to be addressed to ensure the responsible and ethical use of AI in clinical settings. Despite these challenges, the emerging framework of AI integration with clinical settings has the potential to revolutionize cancer care and result in optimized, personalized, and precise treatment strategies for cancer patients.
4. Discussion
The clinical application of AI in cancer management has immense potential to transform patient care and improve outcomes. The present systematic literature review identified 24 relevant studies that explored the clinical application of AI-based methods in cancer treatment. The studies were categorized based on their primary focus on AI applications in cancer therapeutics, including drug discovery, precision medicine, and treatment optimization in clinical settings. The results of the review demonstrate the potential of AI to improve treatment response prediction, optimize treatment plans, and predict treatment-related adverse events in cancer patients.
Several studies have demonstrated the application of machine learning in early cancer diagnosis and prognosis (57). However, one remaining key challenge in cancer treatment is accurately predicting individual patient responses to various and distinct therapies. Accurate prediction of patient responses to cancer treatment can be based on established cause-and-effect interactions or on substantial associations identified in large sets of relevant data (58). While current treatment recommendations are based on factors such as cancer type, size, stage, and patient characteristics, they may not accurately predict clinical outcomes for each patient. However, AI-based algorithms have emerged as a promising solution to this challenge. By analyzing a patient's unique genetic and molecular profile, AI algorithms can predict treatment responses accurately. This personalized approach has the potential to significantly improve patient outcomes and enhance their quality of life.
In addition to predicting treatment responses, AI can play a crucial role in predicting and alleviating treatment-related adverse events. Chemotherapy, for example, can cause side effects that vary from patient to patient and can significantly impact a patient's quality of life and overall survival. Using AI-based tools to analyze patient data, clinicians can better predict and manage potential adverse events and treatment-related toxicity, eventually developing more personalized and tailored treatment regimens (59, 60). This systematic review identified studies that developed AI methods for predicting treatment-related adverse events, such as chemotherapy toxicity and vomiting, which can aid in minimizing these risks and optimizing treatment plans.
The integration of AI in cancer treatment can lead to more personalized and precise treatment plans for patients. With the aid of computational methods, complex patient data can be analyzed to identify signatures and predict treatment responses. This personalized approach has the potential to improve overall survival rates and quality of life for cancer patients (61). Machine learning and deep learning can be utilized to gather deep-level data in genomics, transcriptomics, proteomics, radiomics, digital pathological images, and other data. These data can provide clinicians with a comprehensive and holistic view of the patient and tumor (62).
Consistent with the current study's findings, other review studies in this area have also shown that AI plays a key role in predicting patient outcomes and personalizing cancer treatment. By analyzing high-dimensional datasets, AI can predict prognosis, side effects, and treatment responses, which helps clinicians make decisions (63, 64). In pancreatic cancer, AI helps with early screening and predicts survival time, recurrence risk, and therapy response, all of which are important for improving patient outcomes (65). Additionally, AI makes it easier to develop predictive models for assessing prognosis and treatment responses in different cancer types (66).
The use of AI capabilities in clinical settings can bring challenges and ethical concerns, such as issues related to the transparency of treatment, concerns about the accountability of the healthcare system, and patient privacy and data security (67). In addition, this tool could potentially affect the physician-patient relationship, and the risk of displacing healthcare professions is another concern about the use of AI in the healthcare system (68). To minimize ethical concerns in the use of AI in clinical care settings, careful consideration of ethical issues and the design of robust regulatory frameworks and strategies seem essential.
The present study highlighted studies that developed prediction models based on peripheral blood immune cell markers and MRI radiometric-based machine learning models to estimate patient responses to immunotherapy. AI-based models have also been developed for the estimation of treatment responses to both novel and conventional therapeutics.
4.1. Conclusions
In conclusion, this systematic literature review provides valuable insights into the current state of AI in cancer therapeutics and its potential impact on the future of cancer treatment. The findings suggest that AI has the marked potential to transform cancer therapeutics by enabling personalized and targeted therapies, improving treatment response prediction, and reducing therapeutic-associated adverse events. However, the successful implementation of AI in cancer therapeutics requires addressing challenges and limitations related to data quality, regulatory approval, and ethical considerations. Future research and collaboration between interdisciplinary teams are required to surmount these challenges and grasp the complete potential of AI in cancer therapeutics.
The incorporation of AI in cancer care necessitates a robust and adaptable structure for health policy. That structure must address ethical concerns, such as data protection, algorithm bias, and equitable access to AI-powered technology. Policymakers must ensure the responsible development and use of AI systems, with a focus on transparency, accountability, and patient safety. Clear guidelines for data sharing, algorithm testing, and AI tools' integration in a clinical environment must be in place. Policies should also enable continuous learning and adaptation of AI tools, creating a culture of innovation and improvement in cancer care with a safeguard for prioritizing patient care.
Although AI capabilities can be effective in the care and treatment of diseases such as cancer, conducting robust clinical trial studies under controlled conditions seems essential to test the processes of using AI in healthcare and treatment and to determine the impact of this technology. In addition, given that the number of studies included in the present study was limited and considering the increasing number of studies that address the use of AI in healthcare, it is recommended to conduct new review studies to investigate the role of AI in the treatment of various diseases.
4.2. Limitations
The present study had several limitations: First, we only included studies in English, and it is possible that some relevant studies in other languages were excluded from our review. Second, the number of articles included in the present study was limited, which could be due to the last search date of December 2023 and the search being conducted in only three databases: PubMed, Scopus, and Web of Science. Finally, although two independent reviewers carefully conducted the screening and quality assessment of the included studies, the potential for bias still exists.