1. Background
According to the World Health Organization, approximately 6 billion people are overweight, and about 1.4 billion people engage in very little physical activity (PA), classifying them as part of the passive group (1). It has been suggested that the prevalence of metabolic syndrome (MetS) is strongly associated with central obesity (2). The MetS is also characterized by other cardiovascular risk factors, including triglyceride (TG) levels above 150 mg/dL, HDL levels below 40 mg/dL in men and below 50 mg/dL in women, fasting blood sugar greater than 110 mg/dL, and blood pressure above 130/85 (3-5). The development of MetS is closely linked to modern urban lifestyles, Western dietary habits, and low PA.
Previous research has shown a prevalence of MetS of approximately 42% in the United States, 39% in Indonesia, 29% in the Netherlands, 31% in China, and 30% in India (6-9). A high prevalence of MetS has also been reported in Iran (10, 11). It is suggested that high intakes of unsaturated fatty acids, fruits, and vegetables, along with moderate consumption of saturated fats, carbohydrates, and red meat, can reduce the risk of MetS (12).
The Mediterranean diet (MD) includes large amounts of vegetables, fruits, legumes, grains, fish, and a high ratio of unsaturated to saturated fatty acids, with moderate dairy consumption and less sugar, meat, and poultry. Studies have shown that individuals adhering to the MD have a lower incidence of MetS compared to those consuming more carbohydrates and fats (13, 14). Mahdavi-Roshan et al. found that overweight or obese individuals had average adherence to the MD, with only 6% highly compliant (15). In contrast, Ramezan et al. reported that despite higher intake of fiber and olive oil, the risk of type 2 diabetes remained high in individuals with higher MD adherence scores (16). Another study also found that while MD adherence was relatively good, there was no significant association with MetS criteria (17).
In addition to diet, PA is a key factor in reducing MetS. Many studies have explored the relationship between MetS, PA, and diet. Mohammadi et al. found that MetS prevalence was higher in obese and overweight individuals compared to those with normal weight. Additionally, the inactive group had significantly higher TGs and higher systolic and diastolic blood pressure than the active group (18). Other studies showed that self-reported PA, measured with the Minnesota Questionnaire, was higher in individuals who consumed polyunsaturated fatty acids, omega-3, and alpha-linolenic acid, and lower in those who consumed more saturated fatty acids (19). A recent European longitudinal study also demonstrated that patients adhering to low-calorie MD recommendations and increased PA showed significant improvements in body weight, waist circumference, Body Mass Index (BMI), HDL cholesterol, TGs, and blood pressure after one year (20). However, some studies found no significant association between nutritional quality indicators and MetS markers after adjusting for PA (21, 22).
Despite numerous studies, regional lifestyle differences in Iran may contribute to an asymmetric incidence of MetS. Moreover, no studies have simultaneously examined PA and MD in adults with MetS across multiple Iranian cities.
2. Objectives
This study aims to investigate the association between PA, adherence to the MD, and MetS among adult men and women in Iran who have been diagnosed with MetS. We hypothesized the following: (1) Higher PA levels will be associated with a lower prevalence of MetS; (2) greater MD adherence will reduce the prevalence of MetS; (3) There will be a significant interaction effect between PA levels and 4-MD adherence on the prevalence of MetS.
3. Methods
3.1. Study Design
This study was conducted following the Declaration of Helsinki, as established by the World Medical Association, and was approved by the Ethics Committee of the University of Mohaghegh Ardabili (protocol code: IR.UMA.REC.1401.075). The study utilized a cross-sectional design to evaluate the prevalence of MetS and its associated factors among individuals over 30 years old. The data were collected from January to May across five cities in Iran. The cities were selected using random sampling, and within each city, clinics were randomly selected for participant recruitment, ensuring diverse representation across different healthcare facilities (cluster sampling). However, due to constraints such as time, resources, and accessibility, convenience sampling was ultimately employed for the final selection of clinics. This meant that data collection was limited to clinics that were accessible and willing to participate.
3.2. Participants
The participants were aged over 30 years, divided into two age groups: Thirty to sixty years old and over 60 years old. A total of 976 responses were collected from five cities: Ardebil, Tehran, Char Mahal-o-Bakhtiari, Kermanshah, and Hamedan. Inclusion criteria for the study required participants to have at least three MetS symptoms. The sample size was determined using the Cochran formula, which calculated the initial sample size to be 900 responses. This ensured the study had sufficient statistical power to assess the prevalence of MetS.
3.3. Data Collection
In the first phase of data collection, participants' height, weight, and waist circumference were measured. Then, questionnaires were distributed, and the average time to complete the questionnaire was 15 minutes. After collecting the responses, we screened the data, and 692 responses were excluded due to having one or two MetS criteria. We also excluded 30 additional responses that were either incomplete or lacked informed consent. In the final analysis, 284 responses, which met at least three MetS criteria, were included.
3.4. Mediterranean Diet Measurement
The MD Adherence Questionnaire was used to verify dietary intake. This questionnaire had 14 questions. The questionnaire ranges from 1 to 14. Scores from 1 to 5 correspond to low compliance (inappropriate dietary patterns), 6 to 9 correspond to moderate compliance, and scores from 10 or more are considered high adherence (healthy eating pattern) (23).
3.5. Physical Activity Measurement
The International PA Questionnaire-Short Form (IPAQ-SF) was used in this study. It contains seven questions divided into four PA categories: Sitting, walking, moderate-intensity activity, and high-intensity activity. All items reflect activities performed during the previous seven days. We estimated the total amount of PA in MET-min/week and the time spent sitting (24). In this study, the Iranian version of the IPAQ-SF was used. The validity and reliability of the IPAQ-SF have been well-established in the Iranian population. A study demonstrated that the IPAQ-SF had high reliability (Cronbach’s alpha = 0.7, Spearman Brown correlation coefficient = 0.9) and valid measurement properties when used with Iranian participants (25). To calculate each person's energy consumption based on PA, the energy consumed for each activity (MET) was multiplied by the activity's duration (in minutes) and the number of days in the week. The amount of MET was 3.3 for walking, 4 for moderate PA, and 8 for vigorous PA. A combined total PA MET-min/week was computed as the sum of walking + moderate + vigorous MET-min/week scores.
According to MET scores, three levels were estimated for participants' PA:
1. Vigorous: (A) Vigorous-intensity activity on at least three days, achieving a minimum of at least 1500 MET-minutes/week or seven or more days of any combination of walking, moderate-intensity, or (B) vigorous-intensity activities achieving a minimum of at least 3000 MET-minutes/week.
2. Moderate: (A) Three or more days of vigorous activity of at least 20 minutes per day, (B) five or more days of moderate-intensity activity or walking of at least 30 minutes per day, or (C) five or more days of any combination of walking, moderate-intensity, or vigorous-intensity activities achieving a minimum of at least 600 MET-min/week.
3. Light: Those individuals who do not meet the criteria for the other categories.
3.6. Metabolic Syndrome Measurement
We used the Japanese Questionnaire Model (JAMRISC) in the present study. This questionnaire contains 11 items, including age, gender, waist circumference, PA and nutritional habits, smoking, history of hypertension, diabetes, and myocardial/cerebral infarction (26). According to the questionnaire’s guidelines, we set the cutoff point at 20. If the participant’s score was lower than 20, they were classified as “no risk”, and if the participant’s score was higher than 20, they were classified as “at risk”. The calculation formula for the total risk score with the JAMRISC Questionnaire is:
[1.3369 × gender (male = 1, female = 0)] + (0.1897 × abdominal circumference cm) + [1.3738 × history of hypertension (yes = 1, no = 0)] + [1.5084 × history of hyperglycemia / urinary sugar (yes = 1, no = 0)] + [0.8768 × exercises (less than 2 h = 1, 2 h or more = 0)]. According to the questionnaire’s guidelines, we set the cutoff point at 20. If the score was lower than 20, it was classified as “no risk”, and if the score was higher than 20, it was classified as “at risk”.
3.7. Statistical Analysis
All statistics were completed using IBM SPSS® Statistics Version 22 software. The Shapiro-Wilk test assessed dependent variables for normality. Frequencies, medians, and standard deviations were performed for the primary descriptor. An independent t-test sample was performed on the groups' baseline characteristics to assess any differences. Detailed variables and a one-way analysis of variance (ANOVA) were performed depending on the number of categories of each variable. The alpha level for this study was set at 0.05. If a significant interaction was found, a least significant difference post hoc test was performed to evaluate where the difference was. A one-way multivariate analysis of variance (MANOVA) was performed to examine whether the city determined the results for TM, MetS, and MET-minutes/week, taken together as three dependent variables. Levene’s test was used to check homoscedasticity. A correlational value above 0.5 was considered to be strong, values above 0.50 were considered high, 0.3 - 0.49 were considered moderate, and any value less than 0.29 was considered to be poor (27).
4. Results
Table 1 demonstrates the frequency of cities and characteristics of participants. According to the data presented, 10.9% of participants were from Ardebil, 28.9% were from Tehran, 10.9% were from Char Mahal-o-Bakhtiari, 32.4% were from Kermanshah, and 16.9% were from Hamedan. The average age of participants was 51 years, with an average weight of 84.29 kg. The data revealed that 45% of participants had diabetes (60% women, 40% men), 63% had hypertension (63% women, 37% men), and 56% had hyperlipidemia (62.5% women, 37.5% men). Among those with diabetes, 81.1% were aged 30 - 60, and 18.9% were over 60. Similarly, 81.2% of participants with hypertension were between the ages of 30 and 60, and 18.8% were over 60 years old. For hyperlipidemia, 77.4% were aged 30 - 60, and 22.6% were over 60 years old.
| Cities | No. (%) or Mean ± SD |
|---|---|
| Ardebil | 31 (10.9) |
| Tehran | 82 (28.9) |
| Chahar Mahal-o-Bakhtiari | 31 (10.9) |
| Kermanshah | 92 (32.4) |
| Hamedan | 48 (16.9) |
| Disease | |
| Diabetes | 128 (45) |
| Hypertension | 158 (63) |
| Hyperlipidemia | 179 (56) |
| Characteristics | |
| Age | 51.42 ± 11.57 |
| Height | 165.15 ± 9.92 |
| Weight | 84.29 ± 12.78 |
| BMI | 0.0031 ± 0.0003 |
| Waist circumference | 105.39 ± 13.79 |
Frequency of Cities, Disease, and Characteristics of Participants
4.1. Differences Between Dependent Variables
4.1.1. Differences Between Dependent Variables Based on Gender
We hypothesized that there are significant differences in PA levels, MetS, and MD based on gender. Table 2 demonstrates MD, PA, and MetS based on gender. We used an independent t-test sample to assess differences between gender groups on PA, MetS, and MD. According to our results, men (21.55 ± 6.59) had significantly higher scores in MetS than women (18.81 ± 8.1), t (253.65) = -3.10, P ≤ 0.001. However, we found no difference between men and women in PA and MD.
| Variables | Mean ± SD | Leven’s Test | t-Test | 95% Confidence Interval of the Difference | Cohen’s D | |||
|---|---|---|---|---|---|---|---|---|
| F-Value | P-Value | t | P-Value | Lower | Upper | |||
| MetS | 6.58 | < 0.001 | -2.94 | < 0.001 a | -4.4844 | -1.0025 | 0.37 | |
| Male | 21.55 ± 6.59 | |||||||
| Female | 18.81 ± 8.1 | |||||||
| MD | 0.04 | 0.83 | 0.79 | 0.42 | -0.50323 | 1.19032 | 0.17 | |
| Male | 8.79 ± 3.45 | |||||||
| Female | 8.44 ± 3.52 | |||||||
| PA | ||||||||
| Vigorous-MET b | 4.69 | 0.03 | 1.19 | 0.23 | -200.6821 | 820.3363 | 0.16 | |
| Male | 918.09 ± 2401.77 | |||||||
| Female | 608.26 ± 1454.04 | |||||||
| Moderate-MET b | 0.46 | 0.49 | 0.54 | 0.58 | -222.3246 | 394.2512 | 0.06 | |
| Male | 566.85 ± 1480.72 | |||||||
| Female | 480.89 ± 1136.11 | |||||||
| Light-MET b | 2.38 | 0.12 | 1.62 | 0.10 | -23.4938 | 249.2897 | 0.19 | |
| Male | 454.45 ± 624.49 | |||||||
| Female | 341.55 ± 524.90 | |||||||
| Total-Mets | 4.81 | 0.02 | 1.39 | 0.16 | -211.1668 | 1228.5434 | 0.18 | |
| Male | 1939.40 ± 3342.51 | |||||||
| Female | 1430.72 ± 2174.31 | |||||||
Physical Activity, Mediterranean Diet, and Metabolic Syndrome According to Gender
4.1.2. Differences Between Dependent Variables Based on Age
We hypothesized that there are significant differences in PA levels, MD, and MetS based on age. Table 3 demonstrates, using an independent samples t-test, the results obtained for MD, PA, and MetS, depending on age. Our results indicated that individuals aged 30 - 60 years had significantly higher scores in vigorous-MET (898.54 ± 2077.52 vs. 118.75 ± 339.10), t (253.55) = 5.32, P ≤ 0.001, moderate-MET (634.90 ± 1414.41 vs. 92.50 ± 296.93), t (268.92) = 5.30, P ≤ 0.001, and total-METs (1904.70 ± 2928.87 vs. 635.97 ± 993.02), t (276.29) = 5.44, P ≤ 0.001, compared to those over 60 years. Individuals over 60 years (9.78 ± 2.59) had higher MD adherence compared to those aged 30 - 60 years (8.22 ± 3.64), t (142.60) = -3.82, P ≤ 0.001. MetS was not different between age groups (P > 0.05).
| Variables | Mean ± SD | Leven’s Test | t-Test | 95% Confidence Interval of the Difference | Cohen’s D | |||
|---|---|---|---|---|---|---|---|---|
| F-Value | P-Value | t | P-Value | Lower | Upper | |||
| MD (y) | 5.05 | 0.25 | -3.826 | < 0.001 a | -2.3637 | -0.7533 | 0.49 | |
| 30 - 60 | 8.22 ± 3.64 | |||||||
| Over 60 | 9.78 ± 2.59 | |||||||
| PA | ||||||||
| Vigorous-MET (y) b | 21.25 | < 0.001 | 5.32 | < 0.001 a | 491.5983 | 1067.9925 | 0.64 | |
| 30 - 60 | 898.54 ± 2077.52 | |||||||
| Over 60 | 118.75 ± 339.10 | |||||||
| Moderate-MET (y) b | 29.21 | < 0.001 | 5.30 | < 0.001 a | 340.9415 | 743.8766 | 0.63 | |
| 30 - 60 | 634.90 ± 1414.41 | |||||||
| Over 60 | 92.50 ± 296.93 | |||||||
| Light-MET (y) b | 1.35 | 0.24 | -0.66 | 0.50 | -211.6687 | 104.7281 | 0.09 | |
| 30 - 60 | 371.25 ± 543.53 | |||||||
| Over 60 | 424.72 ± 637.50 | |||||||
| Total-MetS (y) | 23.88 | < 0.001 | 5.44 | < 0.001 a | 809.5842 | 1727.8842 | 0.64 | |
| 30 - 60 | 1904.70 ± 2928.87 | |||||||
| Over 60 | 635.97 ± 993.02 | |||||||
| MetS (y) | 0.32 | 0.12 | -1.43 | 0.15 | -3.7064 | 0.5835 | 0.21 | |
| 30 - 60 | 19.47 ± 7.89 | |||||||
| Over 60 | 21.03 ± 6.84 | |||||||
Physical Activity, and Mediterranean Diet, and Metabolic Syndrome According to Age
4.1.3. Differences Between Dependent Variables According to Mediterranean Diet Levels
We hypothesized that there are significant differences in PA and MetS according to MD levels. Table 4 demonstrates PA and MetS depending on MD levels. According to the data presented, there was no significant difference between MD levels in MetS and total PA. However, those who had a low MD planned their light MET (651.75 ± 828.08) better than moderate MET (316.77 ± 500.12) and vigorous MET (367.40 ± 513.27), F (3,280) = 5.03, P ≤ 0.001.
| Variables | No. | Mean ± SD | F-Value | P-Value | Eta Squared |
|---|---|---|---|---|---|
| Vigorous-MET a | 0.01 | 0.88 | < 0.001 | ||
| High | 135 | 698.96 ± 2202.92 | |||
| Moderate | 113 | 705.48 ± 1554.76 | |||
| Low | 36 | 866.66 ± 1317.38 | |||
| Moderate-MET a | 1.20 | 0.30 | < 0.001 | ||
| High | 135 | 392.00 ± 999.18 | |||
| Moderate | 113 | 640.70 ± 1631.16 | |||
| Low | 36 | 563.33 ± 803.10 | |||
| Light-MET a | 5.03 | < 0.001 b | 0.03 | ||
| High | 135 | 367.40 ± 513.27 | |||
| Moderate | 113 | 316.77 ± 500.12 | |||
| Low | 36 | 651.75 ± 828.08 | |||
| Total-Mets | 0.79 | 0.45 | 0.005 | ||
| High | 135 | 1458.36 ± 2717.11 | |||
| Moderate | 113 | 1662.96 ± 2744.23 | |||
| Low | 36 | 2081.75 ± 2245.16 | |||
| MetS | 1.12 | 0.32 | 0.82 | ||
| High | 135 | 19.44 ± 7.87 | |||
| Moderate | 113 | 19.72 ± 7.57 | |||
| Low | 36 | 21.5907 ± 7.28 |
Physical Activity, Metabolic Syndrome, and Adherence to Mediterranean Diet
4.1.4. Differences Between Dependent Variables According to Physical Activity Levels
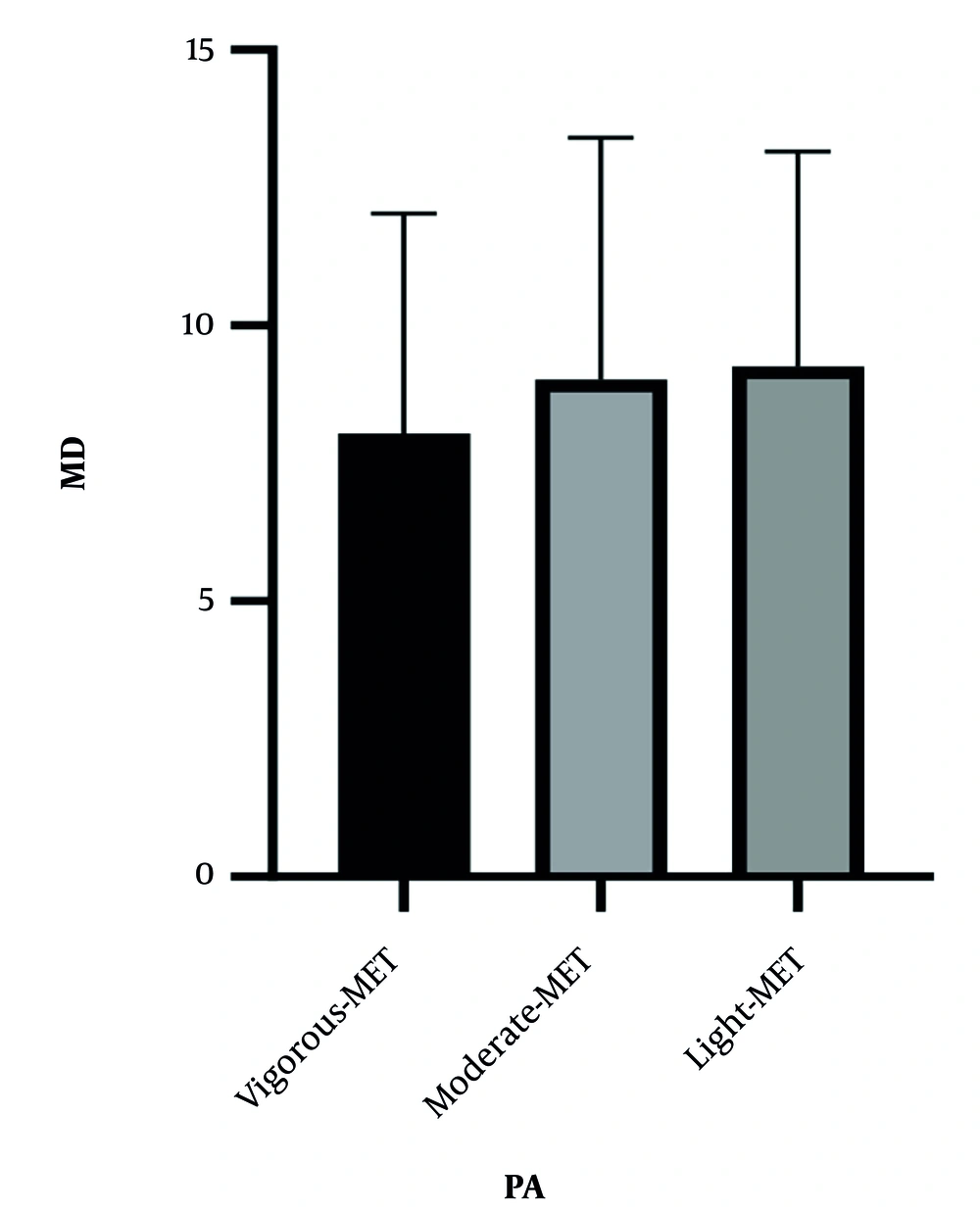
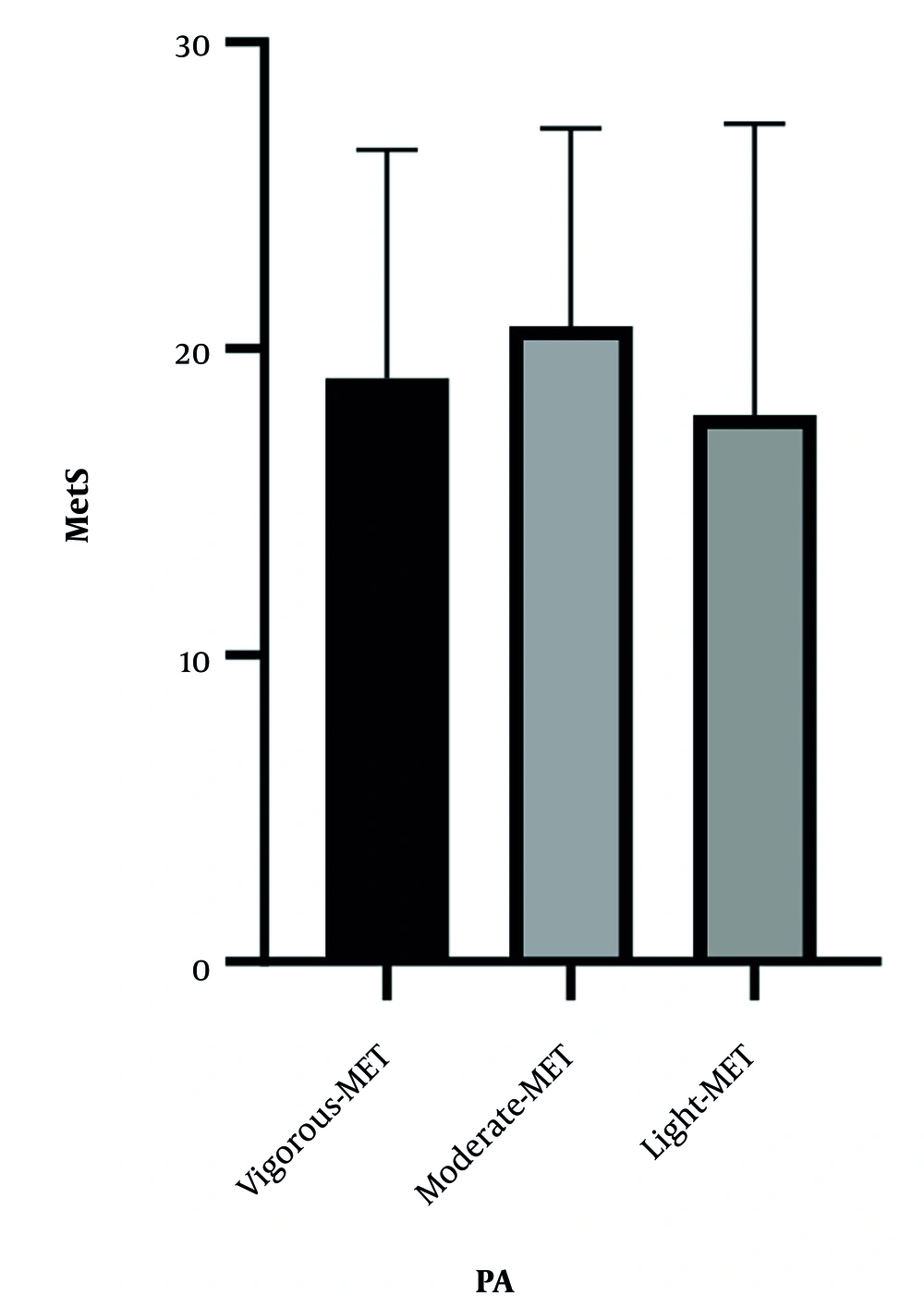
We hypothesized significant differences in MD and/or MetS according to PA levels. Figures 1 and 2 demonstrate the relationship between MD and MetS based on PA levels. According to the data presented, no significant differences were observed across different PA levels (MD; F (2,281) = 1.18, P = 0.30, MetS; F (2,281) = 1.25, P = 0.28).
4.2. Correlations Between Variables
We hypothesized that there is a significant correlation between dependent variables. Table 5 shows the correlations between the variables studied. Our results demonstrate there was a significant relationship between MD and light PA, r = -0.142, P = 0.016; vigorous and moderate PA, r = 0.266, P ≤ 0.001; vigorous and light PA, r = 0.141, P = 0.01; vigorous PA and total PA, r = 0.854, P ≤ 0.001; moderate PA and light PA, r = 0.118, P = 0.04; moderate PA and total PA, r = 0.687, P ≤ 0.001; and light PA and total PA, r = 0.366, P ≤ 0.001.
4.3. The Determination of Results and the City Predictor
We hypothesized that the results for PA, MD, and MetS are determined by the city of residence. A one-way MANOVA was performed to examine whether the results for the MD, MetS, and MET minutes were determined by the city (Table 6). The results indicated that the city (Wilk’s lambda = 0.833, F = 4.363, P ≤ 0.001, partial Eta squared = 0.059) had a significant relationship with the combination of dependent variables. Follow-up ANOVA tests showed that the city of residence had a significant effect on MD adherence (F = 5.907, P = 0.00, η2 = 0.06) and PA (F = 4.087, P = 0.00, η2 = 0.04). However, differences in MetS were not statistically significant across cities (F = 1.981, P = 0.09, η2 = 0.02).
| Effects | Value | F-Value | Hypothesis DF | Error DF | P-Value | Partial Eta Squared |
|---|---|---|---|---|---|---|
| Intercept | ||||||
| Pillai's trace | 0.924 | 1125.583 b | 3.00 | 277.00 | < 0.001 | 0.92 |
| Wilks' lambda | 0.076 | 1125.583 b | 3.00 | 277.00 | < 0.001 | 0.92 |
| Hotelling's trace | 12.190 | 1125.583 b | 3.000 | 277.000 | < 0.001 | 0.924 |
| Roy's largest root | 12.190 | 1125.583 b | 3.000 | 277.000 | < 0.001 | 0.92 |
| City | ||||||
| Pillai's trace | 0.172 | 4.231 | 12.000 | 837.000 | < 0.001 | 0.05 |
| Wilks' lambda | 0.833 | 4.363 | 12.000 | 733.165 | < 0.001 | 0.059 |
| Hotelling's trace | 0.194 | 4.468 | 12.000 | 827.000 | < 0.001 | 0.06 |
| Roy's largest root | 0.159 | 11.093 c | 4.000 | 279.000 | < 0.001 | 0.13 |
| Post-hoc ANOVA results for dependent variables | ||||||
| Dependent variable | ||||||
| MD | - | 5.907 | - | - | < 0.001 | 0.06 |
| MetS | - | 1.981 | - | - | 0.09 | 0.02 |
| PA | - | 4.087 | - | - | < 0.001 | 0.04 |
Multivariate and Post-hoc Analysis of Variance Test of Variance Between the Three Dependent Variables and the City a
5. Discussion
Our study aimed to determine the correlation between the MD, MetS, and PA variables in adults with MetS in Iran. In summary, our findings showed that there were no significant gender differences in PA and MD, while men had significantly higher MetS risk scores compared to women. The younger age group exhibited significantly higher scores in vigorous, moderate, and total PA compared to the older age group, while older adults showed higher adherence to MD compared to younger adults. At the same time, no significant differences in MetS were observed across age groups. Individuals with low MD scores had higher levels of light PA compared to those with moderate or high MD scores. However, we did not observe any significant differences in MetS or overall PA. There was no significant relationship between MD and MetS based on PA levels. Moreover, a significant correlation was found between PA levels and MD, as well as light PA. No substantial correlation was observed between MetS and PA/MD. Finally, the results of the study may vary depending on the participants' city of residence. This is the first study to compare the dependent variables (PA, MD, and MetS) across multiple cities. It has been well-established that there is a positive relationship between waist circumference and higher levels of body mass, body fat percentage, and BMI (22). These factors are associated with MetS (5). Previous studies have demonstrated that the prevalence of MetS may vary by gender (22, 28-30). Higher TG levels, lower low-density lipoprotein, higher BMI, and higher waist circumference are the most important predictors of MetS prevalence (22, 28).
In the present study, we observed that men were at higher risk of MetS than women. While no significant differences were found in waist circumference, self-reported hypertension, and self-reported hyperlipidemia between genders, men showed a higher mean weight and an overall higher risk of MetS. Additionally, our study revealed that the prevalence of self-reported diabetes was significantly higher in men, a finding consistent with international studies suggesting that men are more prone to developing MetS-associated comorbidities, such as diabetes (31). These differences may be attributed to a combination of biological, hormonal, and lifestyle factors. Insulin resistance, or its associated condition, hyperinsulinemia, directly contributes to other metabolic risk factors and is also a key factor in the development of type 2 diabetes. However, identifying the unique role of insulin resistance is complicated by the fact that it is closely linked to obesity. Therefore, further investigation is needed to determine whether insulin resistance specifically causes other metabolic risk factors in men.
We also observed no significant difference in PA or MD adherence between genders. Recently, a cohort study reported no significant difference in Mediterranean lifestyle adherence between genders in individuals at high risk for MetS (13). Contrary to our findings, past studies have indicated that men are generally more physically active and have better adherence to the MD compared to women (15, 32). Another study showed that, although men are more physically active, they may not adhere as well to dietary recommendations, while women often report a more structured eating routine. Despite these observations, we found no significant differences in PA or MD adherence between genders (33). Although these factors are important, it has been observed that other protective lifestyle factors, such as income level, educational attainment, occupation, and other social factors, are also associated with the risk of developing MetS (34, 35). Therefore, future studies should investigate the combination of these factors to better understand their role in the development of MetS.
Moreover, we hypothesized that the prevalence of MetS increases with age, as it has been confirmed that basal metabolism, lean body mass, and energy requirements decrease with age, contributing to obesity (36). Our results demonstrated that although the younger age group was more active than the older age group, there was no significant difference between age groups in terms of MetS. It is possible that our participants over-reported their PA levels. Additionally, our results showed that individuals aged 30 - 60 years had significantly lower scores compared to those over 60 years, with significantly higher average weight, self-reported diabetes, and self-reported hypertension in the younger age group. These results suggest a higher prevalence of metabolic disorders among younger adults in our study. Therefore, interventions should focus on improving diet quality and PA in adults.
We also observed no significant difference between MD levels in MetS or overall PA. However, a significant difference was observed between MD levels in light PA. Indreica et al. showed that participants with higher adherence to MD engaged in more intense PA (37). Our statistical analyses showed no significant correlation between MetS and PA/MD. However, a significant relationship was found between MD and light PA. High adherence to MD has been well-defined as being associated with a lower MetS risk (16, 17, 21, 38-42). Fish, olive oil, and nuts, which are components of MD, are known to reduce the MetS risk (43). Previous studies have reported that low-fat diets enriched with polyunsaturated fatty acids also reduce TG levels (19, 44, 45). Additionally, the consumption of vegetables, olive oil, and Low-Glycemic Index Foods has been shown to lower blood glucose, C-peptide values, fasting serum testosterone levels, and insulin resistance, while increasing serum insulin-like growth factor binding proteins (IGFBP) 1 and 2 levels (46-49). Nitric oxide levels and endothelin-1 are modulated by consuming extra virgin olive oil and nuts (43).
Regular PA is associated with reduced MetS (50-54). This may be due to significant improvements in systolic and diastolic blood pressure, fasting blood glucose, insulin, interleukin 6, and TGs through PA (51, 54). However, we did not find a significant correlation between MetS and MD/PA. More specifically, we found moderate adherence to MD in our study. Seventy-seven percent of our participants consumed pasta or rice at least five days a week, 82% consumed grains or carbohydrates every morning, and 30 to 36% regularly consumed vegetables, olive oil, and fish. Adherence to MD is influenced by sociocultural, religious, and economic factors (29). In Iran, the typical diet includes foods such as rice, bread, vegetables, legumes, yogurt, and meats like lamb and chicken, which share similarities with the MD, particularly in terms of plant-based foods, legumes, and moderate meat consumption. However, the Iranian diet differs in its higher consumption of rice, specific herbs, and spices not commonly emphasized in the MD diet. Also, nuts, extra virgin olive oil, and fish may be difficult for our participants to provide. Additionally, while self-reported data is a commonly used method in large-scale epidemiological studies due to its practicality, participants may have inaccurately reported their responses. Future studies could benefit from incorporating objective measures, such as food diaries for dietary assessment, to minimize recall bias and provide more accurate and reliable data. Furthermore, combining self-reported data with biochemical measurements (e.g., blood tests) could offer a more comprehensive understanding of the relationship between diet and MetS. However, more research is needed to conclude for larger populations.
Finally, we observed that the city of residence of the participants influenced MD, PA, and MetS. To the best of our knowledge, no previous studies have reported on the relationship between these dependent variables and the city of residence in Iran. This finding is consistent with other research demonstrating significant effects of city of residence on MD and PA outcomes (36). Notably, we observed significant differences in MD and PA across cities. These disparities may reflect variations in urban infrastructure, cultural dietary patterns, socioeconomic status, and access to recreational and health-promoting facilities. Such city-specific characteristics likely play a key role in shaping health-related behaviors and outcomes, emphasizing the importance of considering environmental and geographical factors in public health planning.
In contrast, although differences in MetS were observed across cities, these differences did not reach statistical significance. This suggests that MetS may be more strongly influenced by individual-level factors (e.g., genetics, chronic conditions) or may require a larger sample size to detect city-level effects. Therefore, we recommend that future studies use a larger and more representative sample to provide more accurate and generalizable conclusions. Another key strength of this study was the use of a validated MetS Questionnaire, an appropriately explored alternative to blood tests (26). This approach may offer a cost-effective and time-efficient method for MetS assessment. However, further research is needed to validate its effectiveness.
5.1. Conclusions
Our study aimed to determine the correlation between MD, PA, and MetS among adults in Iran. There was no significant difference in MD and PA based on gender, while men reported higher MetS risk scores. Younger adults were more active than older adults, while older adults showed higher adherence to MD. However, no significant difference was observed between younger and older age groups in MetS risk score. MetS was not correlated with PA and MD. City of residence influenced MD, PA, and MetS. It seems that the prevalence of metabolic disorders begins at a younger age. Central obesity and a history of metabolic disorders are the main reasons for the prevalence of MetS in our study. Due to lower PA in older adults and the possibility of MetS from an early age, social policies should encourage the promotion of a Mediterranean lifestyle. Further research needs to be conducted to better understand this topic.
5.2. Limitations
There are a few limitations to our study that should be addressed. Firstly, participants were selected from a specific and accessible population, which may limit the generalizability of the findings to the broader population. Secondly, since only clinics that were accessible and willing to participate were included, the sample may not represent the full diversity of the broader population. Finally, we relied on self-reported PA, which may not accurately reflect actual behavior. Future studies should use more comprehensive random sampling methods to improve the generalizability of the results. Additionally, participants were asked to report their PA levels and dietary habits, and such self-reports may be influenced by factors such as forgetfulness, social desirability bias, or inaccurate recall. Investigating this issue in diverse populations and using accelerometers for more accurate PA assessment or food diaries would be beneficial.