1. Background
Hospital-acquired infections (HAIs) can result in prolonged hospital stays, increased healthcare costs, and elevated mortality rates, thereby impacting both patients and healthcare workers. Airborne transmission plays a crucial role in the spread of HAIs (1, 2). The most important source of hospital infections is bioaerosols, which usually exist as colloidal suspensions in the air, consisting of suspended liquid droplets and solid particles originating from plants, such as pollen, and animals (3, 4). These particles can include living or dead microorganisms such as bacteria, viruses, and fungi (5). The growth and proliferation of bioaerosols in the internal environments of the hospital are increased by various factors such as the type of walls and floors, the location of the hospital building (high traffic or low traffic areas), air change rate, air movement, and season (6). A critical consequence of HAIs is the emergence of multidrug-resistant organisms, which further complicate treatment outcomes. Current data indicate that HAIs affect approximately 3.2% (687,000 or 1 in 31 patients) of hospitalized patients in the United States and 6.5% of patients in the European Union/European Economic Area, with global prevalence likely exceeding these estimates (7). This mode of transmission occurs when pathogens are conveyed from infected individuals to susceptible individuals through particles that remain suspended in the air (8). The transmission of these infections is considered an indirect route and is influenced by various factors, including the presence of an airborne pathogenic organism, the identification of contamination sources, detection in air samples, and specifically, the occurrence of infections in patients exposed to contaminated air (9). Infectious bacteria such as Staphylococcus aureus, Mycobacterium tuberculosis (the causative agent of tuberculosis), Acinetobacter baumannii, Aspergillus, and Pseudomonas aeruginosa have the potential to spread via airborne particles (10). In hospitals, Staphylococcus aureus and Streptococcus pyogenes are two pathogens that may cause severe invasive infections (11). Airborne dissemination of pathogens can occur through different means, such as respiratory droplets, dust particles carrying pathogens, contaminated medical equipment, ventilation system malfunctions, environmental sources, and even hospital personnel and visitors (12, 13). The transmission of airborne infectious diseases is significantly impacted by particle size and dryness. Temperature and relative humidity act as primary environmental factors, playing a critical role in altering transmission rates (14). Following the COVID-19 pandemic, hospitals have been attempting to reduce the risk of airborne infectious disease transmission by increasing the air change per hour (ACH) — the number of times that the total air volume in a room or space is completely removed and replaced in an hour — and by using ventilation systems proportional to the needs of different hospital areas, such as operating rooms and patient rooms (15). In a study conducted by Mirzaei et al. to evaluate isolation rooms in Mashhad hospitals, the results showed that in 81% of cases, the number of air changes per hour and in 72% of cases, the air inlet and outlet standards were not up to standard (16). Fernström and Goldblatt examined the role of aerobiology — the study of airborne organisms — in infectious disease transmission. Their findings indicated that factors such as particle size and type, airborne duration, travel distance, and environmental conditions have significant effects on airborne disease transmission. Furthermore, high-efficiency filtration remains the most common method for reducing airborne particle transmission (17). According to studies by Li et al., achieving around 25 ACH is necessary for rapid aerosol removal, achievable through portable heating, ventilation, and air conditioning (HVAC) air purifiers; however, solely relying on HVAC systems poses challenges, as it reduces the influx of fresh air into hospital spaces (3). In a study conducted by Qiu et al., results demonstrated that bacterial species distribution is influenced by ventilation conditions and humidity levels in the environment, and that ventilation played a more crucial role in controlling bacterial distribution when humidity levels were higher (18). Hassan and Zeeshan, in a study of two public hospitals with different ventilation systems, disinfection protocols, and occupancy levels, found that the most prevalent bacterial species were Staphylococcus species (53% of samples), Micrococcus spp. (30%), and Bacillus spp. (11%), while the predominant fungal species were Aspergillus spp. (67%) and Penicillium (28%) (19). Only a limited number of studies have considered the impact of infection type on other parameters, such as pathogen density and transmission, length of hospital stay, and other factors.
2. Objectives
Given the critical importance of controlling HAIs and the vital role of indoor air quality, this study aims to quantitatively and qualitatively assess the correlation between ventilation systems, bioaerosol density, and the incidence of HAIs, marking a significant step toward improved infection control in hospital environments.
3. Methods
In this descriptive-analytical study, conducted in a 325-bed educational hospital that is 70 years old and located in a high-traffic area of the metropolis of Mashhad, data from 473 inpatients were collected from the hospital's infection control department between October 2021 and June 2024. The information collected included patient age, gender, hospital ward, duration of hospitalization until infection, bioaerosol density in each ward, and infection type. A Lutron PHB318 (Taiwan) was employed to measure air velocity in ventilation inlets and outlets (Figure 1), adhering to ASHRAE standards (Figure 2). An anemometer model TES 1340 (Taiwan) was used to measure airflow speed, in accordance with ACGIH standards. Based on Equation 1, the flow rate into the room was calculated. The ACH were calculated based on Equation 2 (20).
V= Air speed (fpm); A= Area of inlet (ft2)
That Q (ft3/min) = air flow and V= volume of room (ft3)
Bioaerosol sampling was conducted based on the NIOSH 0800 standard method. Air samples were collected using a sampling pump (Air Quick Take 30) equipped with a single-stage Anderson impactor, with a flow rate of 28.3 L/min. Prior to each sampling, all equipment was thoroughly cleaned with 70% isopropyl alcohol. After sample collection, the blood agar plates were incubated at 37°C for 24 to 48 hours. Colony counts were then performed on each plate, and bioaerosol concentration was calculated as colony-forming units per cubic meter (CFU/m3) using Equation 3 (21, 22).
That N is number of bioareosol per plate al L is volume of air sampling liter.
Data analysis was performed using SPSS software version 26. Descriptive statistics for infection type based on hospital ward and patient gender were assessed. The Kruskal-Wallis test was employed to compare the mean infection type with other quantitative parameters, followed by post hoc tests for pairwise comparisons. To examine the association between infection type and gender, as well as between infection type and ward, cross-tabulation and Fisher's exact test were applied. Spearman's correlation was utilized to assess associations between bioaerosol density, ACH, and other quantitative variables.
4. Results
This study aimed to investigate the relationship between ventilation parameters and the incidence of HAIs among 473 patients with infections. Data analysis revealed that the highest number of infected patients were admitted to ICU3. Additionally, the highest bioaerosol concentration was observed in the emergency surgery department. Further data analysis indicated that ICU3 had the highest ACH rate, while the emergency surgery department had the lowest. The relative humidity was highest in ICU2, and the highest ambient temperature was recorded in the men's orthopedic ward (Table 1).
| Value and Section Type | Number of People | Mean | |||
|---|---|---|---|---|---|
| Bioaerosol Density | ACH | Relative Humidity (%) | Temperature (℉) | ||
| ICU 1 | 88 | 320 | 5.50 | 21.3 | 78 |
| ICU 2 | 54 | 156 | 3.8 | 32.5 | 77.1 |
| ICU 3 | 119 | 162 | 13.62 | 23.3 | 76 |
| ICU 4 | 109 | 472 | 4.7 | 21.5 | 78.5 |
| ICU 5 | 25 | 86 | 5 | 27.1 | 76 |
| Men's orthopedic department | 18 | 226 | 3.5 | 25.6 | 79.9 |
| Emergency surgery | 60 | 474 | 3 | 24.7 | 77.7 |
| Total | 473 | 300.14 | 6.7459 | 24.029 | 77.438 |
Abbreviation: ACH, air changes per hour.
Table 2 presents data on the types of HAIs and ACH among male and female patients across different hospital departments. Overall, Acinetobacter-related infections show the highest prevalence, particularly in ICU3, where 141 male and 29 female patients have been infected. Other common infections include Escherichia coli and Pseudomonas aeruginosa, both of which also exhibit high prevalence in ICU3, with E. coli observed in 44 male and 8 female patients, and P. aeruginosa in 43 male and 5 female patients. In the emergency surgery unit, the most frequent infection is Staphylococcus aureus, affecting 37 male and 2 female patients. Conversely, certain infections, such as those caused by Proteus and Serratia, have a lower incidence, appearing only in isolated cases within departments such as ICU1, ICU4, and male orthopedics. The overall patient count indicates that a total of 473 individuals have contracted one of these infections, comprising 403 men and 70 women. Additionally, ICU3 has the highest infection count, highlighting a significant concentration of HAIs within this unit.
| Value and Infection Type | Number of Infected People by Gender | The Highest Number of Infection Species in That Section Type | ||
|---|---|---|---|---|
| Male | Female | No. | Section Type | |
| Acinetobacter | 141 | 29 | 41 | ICU3 |
| Staphylococcus epidermidis | 30 | 3 | 8 | ICU4 |
| Staphylococcus aureus | 37 | 2 | 12 | Emergency surgery |
| Escherichia coli | 44 | 8 | 15 | ICU3 |
| Enterobacter | 31 | 6 | 9 | Emergency surgery |
| Enterococcus | 8 | 1 | 3 | ICU3 |
| Proteus | 2 | - | 1 | ICU4 , Men's Orthopedic Department |
| Serratia | 1 | - | 1 | ICU1 |
| Pseudomonas aeruginosa | 43 | 5 | 17 | ICU3 |
| Citrobacter | 1 | - | 1 | Emergency surgery |
| Candida | 17 | 7 | 12 | ICU3 |
| Klebsiella | 21 | 9 | 11 | ICU1 |
| Klebsiellaoxytoca | 27 | - | 7 | ICU1 , ICU3 , ICU4 |
| Total | 403 | 70 | 138 | 5 |
Based on the results obtained from Fisher's Exact Test in Table 3, a significant relationship was found between the type of infection and the type of department (P = 0.005). However, no significant correlation was observed between the type of infection and gender (P = 0.091). Furthermore, the results of the Spearman test indicated a significant inverse correlation between bioaerosol concentration and relative humidity (P = 0.001), followed by ACH (P = 0.001); however, a significant direct relationship was found between bioaerosol concentration and temperature. No significant correlation was found between ACH and relative humidity (P = 0.228), but a significant inverse relationship was observed between ACH and temperature (Table 4).
| Value | Monte Carlo P-Value (2-sided) |
|---|---|
| Gender | 0.091 |
| Section type | 0.005 |
| Variables | Correlation Coefficient | P-Value |
|---|---|---|
| Relative humidity | ||
| Bioaerosol concentration | -0.43 | 0.000 |
| ACH | -0.055 | 0.228 |
| Temperature | ||
| Bioaerosol concentration | 0.662 | 0.000 |
| ACH | ||
| ACH | -0.447 | 0.000 |
| Bioaerosol concentration | -0.45 | 0.000 |
Abbreviation: ACH, Air changes per hour.
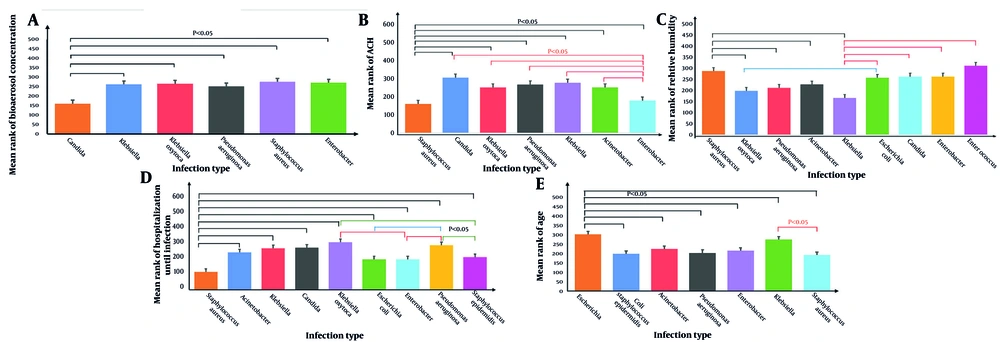
Comparing the average type of infection with other quantitative parameters, most results indicated significant differences between them. According to Figure 3A, the greatest impact of ACH was observed on Candida fungi, while the least impact was on S. aureus bacteria. On the other hand, as shown in Figure 3B, Enterococcus bacteria and subsequently S. aureus exhibited higher prevalence at higher humidity levels, while Klebsiella bacteria were more prevalent at lower humidity levels. S. aureus caused infections in hospitalized individuals within a shorter period, while Klebsiella oxytoca did so over a longer duration (Figure 3C). A greater proportion of younger hospitalized individuals were infected with S. aureus, whereas older individuals were more likely to contract E. coli (Figure 3D). The bioaerosol concentration carrying S. aureus was higher than that of the other infection species, while the lowest concentration of bioaerosols was associated with Candida fungi (Figure 3E). Additionally, no significant difference was found between the type of infection and temperature.
A, The difference between the infection type with bioaerosol concentration; B, the difference between the infection type with air changes per hour (ACH); C, the difference between the infection type with relative humidity; D, the difference between the Infection type with hospitalization until infection; E, the difference between the infection 1.
5. Discussion
This study examined the relationship between ventilation parameters and the prevalence of HAIs in an educational hospital in Mashhad. The findings revealed that infections caused by Acinetobacter spp. exhibited the highest prevalence among both male and female patients, likely due to the pathogen’s remarkable ability to survive and proliferate in hospital environments. These results emphasize the critical role of ventilation systems in controlling bioaerosol transmission and mitigating the risk of HAIs. Furthermore, a statistically significant association was observed between the type of infection and the hospital department, whereas no significant correlation was found between infection type and patient gender. Additionally, bioaerosol concentration demonstrated a significant inverse correlation with both relative humidity and ACH, while exhibiting a direct correlation with ambient temperature. A significant inverse relationship was also identified between ACH and temperature.
A key observation was the inverse correlation between bioaerosol concentration and both relative humidity (P = 0.001) and ACH (P = 0.001), suggesting that enhanced ventilation and optimized humidity levels can effectively reduce airborne microbial loads. Conversely, the direct correlation between bioaerosol concentration and temperature (P = 0.000) suggests that higher ambient temperatures may facilitate microbial proliferation. These findings align with previous research, which underscores the efficacy of increased ACH in mitigating airborne pathogen transmission by diluting contaminated air. Notably, the lowest ACH rate was recorded in the emergency surgery department (3 ACH), which corresponded to the highest bioaerosol concentration, further underscoring the necessity for adequate ventilation in high-risk areas.
The distribution of infection types among different hospital wards revealed that ICU3 exhibited the highest burden of HAIs, particularly infections caused by Acinetobacter spp., Escherichia coli, and P. aeruginosa. This observation is consistent with existing literature highlighting intensive care units as epicenters of multidrug-resistant infections due to prolonged patient stays, the extensive use of invasive procedures, and high exposure to antibiotics. In the study by Choobdar et al., 13.5% of 645 newborns in NICU were identified with HAI (23). Additionally, the emergency surgery department demonstrated a notably high prevalence of S. aureus infections, likely attributable to frequent surgical interventions, high patient turnover, and increased exposure to contaminated surfaces. In alignment with previous studies, this research underscores the role of environmental factors in HAI prevalence (2).
Studies by Rajab (24) have demonstrated that ventilation and filtration systems significantly improve indoor air quality, while research by Shimoda et al. (25) has highlighted the influence of temperature fluctuations on pathogen growth, distribution, and infection control. Furthermore, Qiu et al. (18) emphasized that decreasing relative humidity can adversely affect microbial metabolism, ultimately creating unfavorable conditions for pathogen survival. The results of this study reinforce these findings by demonstrating that Enterococcus spp. and S. aureus thrive in higher humidity conditions, whereas Klebsiella spp. exhibit greater activity under lower humidity levels.
Although recent infection control measures have largely targeted Gram-negative bacteria, particularly those transmitted via direct contact, there has been a notable increase in infections caused by gram-positive bacteria, which display greater resilience in low-humidity environments (26). Centeleghe et al.’s research demonstrated that Klebsiella pneumoniae can persist on dry surfaces for at least four weeks at 55% relative humidity and 21°C, potentially remaining viable in a non-culturable state and contributing to pathogen transmission. This underscores the importance of environmental disinfection and surface decontamination as complementary strategies to ventilation-based infection control measures (27, 28).
The present study also identified a higher bacterial species diversity in ICU wards compared to other hospital departments. This finding may be attributed to the infrequent replacement of air filters, which can serve as microbial reservoirs. If not adequately maintained, air filtration systems may facilitate pathogen proliferation and subsequent reintroduction into hospital environments, exacerbating the risk of airborne transmission. The relatively minimal impact of ACH on S. aureus infections observed in this study can be explained by its primary mode of transmission, which occurs through direct contact and large respiratory droplets rather than airborne dissemination. Thus, while increasing ACH can mitigate airborne infections, effective infection control for S. aureus necessitates a multifaceted approach that integrates stringent surface disinfection and adherence to hygiene protocols. Blanco also found that a 10% increase in relative humidity could increase the prevalence of Methicillin-resistant S. aureus (MRSA) and Vancomycin-resistant enterococci (VRE) by 9 ± 8%.
The relationship between infection type and hospital department can be attributed to various factors, including differences in patients' immune systems, the number of hospitalized patients, traffic of individuals, and the design and organization of departments (29). Demographic analyses indicated no statistically significant association between gender and infection type (P = 0.091), suggesting that environmental and clinical factors play a more influential role in HAI prevalence than patient demographics. However, age-related variations in infection susceptibility were observed. Consistent with previous research, S. aureus infections were more frequently reported among younger patients, while E. coli infections were more prevalent among elderly individuals. The latter finding aligns with established evidence linking E. coli to urinary tract infections (UTIs), which are increasingly common among older adults due to physiological changes and the frequent use of urinary catheters in this population. Escherichia coli is the most common cause of urinary tract infections, with incidence rates between 30 and 100 cases per 1,000 person-years in individuals aged 60 to 90 years (30).
The findings of this study emphasize the necessity of optimizing hospital ventilation conditions to reduce HAIs and enhance healthcare quality. Implementation of improved ventilation strategies, along with the use of high-efficiency particulate air (HEPA) filters and real-time air quality monitoring, could significantly mitigate the airborne transmission of pathogens. Furthermore, future research should explore additional factors influencing HAI prevalence, including air filtration efficiency, disinfection technologies, and the impact of healthcare personnel behaviors on infection control. Addressing these factors not only has the potential to lower healthcare costs and reduce patient hospitalization duration but also to improve overall patient outcomes and enhance the efficiency of healthcare systems.
5.1. Conclusions
This study aimed to determine the relationship between ventilation parameters and the types of infections. The analysis demonstrated statistically significant relationships between bioaerosol concentrations and environmental parameters. Specifically, a strong inverse correlation was observed between bioaerosol concentrations and both relative humidity (P = 0.001) and ACH (P = 0.001), indicating that higher relative humidity and ACH are associated with lower bioaerosol concentrations. On the other hand, a direct correlation was found between bioaerosol concentrations and temperature (P = 0.001), suggesting that warmer conditions contribute to higher bioaerosol levels.
In terms of infection distribution, the type of infection was significantly correlated with the department type (P = 0.005), with Acinetobacter infections being most prevalent (41, 25%), particularly in ICU3. S. aureus was the predominant infection in the emergency surgery department, while Candida infections were significantly influenced by ACH. Notably, S. aureus prevalence increased with higher humidity and was more common in younger patients (P = 0.091 for gender, not significant). The data also revealed that bioaerosol concentrations were highest for S. aureus and lowest for Candida, with temperature not significantly influencing infection distribution.
These findings emphasize the need for controlling environmental factors, such as humidity and ACH, to potentially mitigate bioaerosol-related infection risks in hospital settings. These findings underscore the importance of understanding the types of infections for infection control management in hospitals, which could aid in designing better ventilation systems to prevent HAIs. However, the study had limitations, such as reliance on limited statistical data from a single hospital. Future research is recommended to examine the impact of other environmental factors on bioaerosol density and infection types. Overall, regulating air change rates, controlling temperature and humidity, and understanding infection types appear to be effective strategies for reducing bioaerosol density and minimizing the risk of bacterial and fungal infection transmission in hospital environments.