1. Background
Valproate (2-propylpentanoate) is available as valproic acid (N-dipropylacetic acid), sodium valproate and semisodium valproate, which are widely used as anticonvulsants and mood-stabilizing drugs, primarily in the treatment of epilepsy. Recently, several investigations have proposed its use in the treatment of anxiety, alcoholism, schizophrenia, borderline personality disorder and mood disorders including bipolar disorder (1). It is less commonly used in the treatment of major depression (1, 2). Valproic acid acts on dopamine and glutamine neurotransmission as well as intracellular signaling (3). Valproic acid is marketed under various pharmaceutical preparations and forms. However, the circulating active molecule is the "valproate ion", which is characterized by dose limited absorption, nonlinear plasma protein binding and multiple metabolic pathways of elimination (2). Once absorbed, valproic acid is largely bound to plasma proteins with a relatively small distribution volume (2, 4). Its concentration in cerebrospinal fluid is approximately one-tenth of that in plasma and is directly correlated with the concentration found in tears. At therapeutic ranges (50 - 100 mg/L or 346 - 693 µmol/L) (5), valproic acid half-life varies from 10 to 20 hours in adults, to a significantly shorter duration (6 - 9 hours) in children (4).
Numerous metabolites of valproic acid have been identified since it undergoes extensive liver metabolism. Furthermore, many different evidences suggest that several of the valproic acid metabolites contribute to its pharmacological actions or toxicity (4, 5). Common adverse effects of valproate treatment include weight gain, gastrointestinal symptoms, sedation, tremor, heartburn, impaired vision, hearing loss, respiratory depression, headache, joint pain and mild elevation of liver enzymes (3). Severe hepatotoxicity is rare in adults and many adverse effects are dose related and resolve with dose reduction (3). Overdose in children is usually of accidental origin, whereas in adults it is more likely to be an intentional act. In spite of these effects, use of valproate has the advantage of being easy to manage and it is also well tolerated in long term among patients. In severe intoxications, hemoperfusion or hemofiltration can be an effective means of quick elimination of the drug from the body.
2. Objectives
Determination of valproic acid in serum is required in epilepsy therapy for efficient control of seizures and its serum monitoring is often accomplished by using different techniques. Literature reviews indicate that many different methods including high performance liquid chromatography , gas chromatography and immunological assays for valproic acid determination in patients serum are purposed (6-10). Most of the procedures prior to HPLC separation are complicated and time consuming. Furthermore, several GC analysis with flame ionization detector use different solvents as well as solid phase extraction columns and various internal standards (7). In order to minimize valproic acid adverse effects and maximize its therapeutic activity, the determination of the serum/plasma concentration is critical and the utilization of a rapid, sensitive, simple and cost effective method for its therapeutic monitoring is necessary (11). In the present study we developed, compared, and evaluated valproic acid determination in serum samples by GC and HPLC methods for diagnostic and monitoring purposes of patients under treatment with valproic acid.
3. Materials and Methods
Two systems (The Agilent 1260 Infinity HPLC-Chip/MS system, Agilent Technologies, Santa Clara, California, the USA for HPLC assays and the Agilent 6890 GC, Agilent Technologies, Santa Clara, California, the USA, system for GC assays) were used. Samples assayed were patients' serum and Chromsystems, Chromsystems GmbH, Munich, Germany, as a serum quality control material. All chemicals had a grade suitable for analysis.
3.1. Instruments Set-up
3.1.1. HPLC
The HPLC Agilent 1260 Infinity Series with a C18 column (15 cm length) and inside diameter of 4.6 mm was used. The analysis was performed at 40 °C. The UV absorption spectrometer was used as a HPLC detector at a detection wavelength of 210 nm. The mobile phase consisted of 0.02 M phosphate buffer (pH = 3.0) and acetonitrile-water 65:35 (v/v) that were filtered separately before mixing. A flow rate of 1 mL/min was selected and the internal standard solution was 20 µg/mL butabarbital (6).
3.1.2. Gas Chromatography (GC)
Gas chromatography was performed on the Agilent 6890N GC System, with flame ionization detector and a HP-1 column in (30 m x 0.53 mm). The initial temperature of the column oven was 80 °C, with nitrogen carrier gas. A flow rate of 15 mL/min and a split of 1/10 were selected. The internal standard solution was 20 µg/mL n-caproic acid (7).
3.2. Extraction Procedures
3.2.1. Valproic Acid ((HPLC))
A volume of 200 µL of either serum/control material (three levels) was added to the test tubes. Afterword, 50 µL of internal standard (20 µg/mL butabarbital) and 200 µL of HCl 1N were added and mixed on a vortex mixer for 5 seconds. Later, 500 µL of dichloromethane were added and mixed on a vortex mixer for 30 seconds, then centrifuged at 3000 rpm for 10 minutes. The organic layers were taken and added to another set of test tubes and evaporated at 400 °C with nitrogen gas. Then, the content of the tubes was dissolved with 50 µL methanol and 20 µL of the resulting product were injected for analysis. The peaks were visible at 4.1 minutes for internal standard and at 13.6 minutes for valproic acid.
3.2.2. Valproic Acid (GC)
A volume of 200 µL of either serum/control material (3 levels) was added to the test tubes, followed by 50 µL of internal standard (20 µg/mL n-caproic acid) and 200 µL HCl 1N which were mixed on a vortex mixer for 5 seconds. Later, 200 µL of chloroform was added and mixed on a vortex mixer for 30 seconds, then centrifuged at 3000 rpm for 10 minutes and finally, 1 µL of the organic layers was injected. The peaks were visible at 2.8 minutes for internal standard and at 4.1 minutes for valproic acid.
3.3. Comparison and Evaluation Studies
3.3.1. Precision Studies
We performed intra and inter-assays precision evaluation of the two systems by first reconstituting and then pooling three vials (three levels) of chromsystems quality control materials. Intra-assay precision was determined by analyzing a pool serum sample (each level; n = 10X) in one day. Inter-assay precision was determined by analyzing a pool serum in 20 days.
3.3.2. Recovery Studies
Stock valproic acid standard solution (1000 µg/mL) in methanol was prepared and was kept in the freezer (-20 °C). Then, serum samples with blank and 75 µg/mL valporic acid concentrations were prepared and the recovery percentage in both systems was calculated.
3.3.3. Limit of Quantification
The limit of quantification (LOQ) is the lowest concentration of analyte in a sample that can be determined with acceptable precision and accuracy. Furthermore, LOQ is quoted (12, 13) as the concentration yielding a signal-to-noise ratio of 10:1 and confirmed by analyzing a number of samples near this value (n = 5).
3.3.4. Linearity Studies
For linearity studies, valproic acid concentrations of 25 µg/mL, 50 µg/mL, 100 µg/mL, 200 µg/mL and 400 µg/mL were added to neat pool serums and analyzed in both systems.
3.3.5. Correlation studies
For this purpose, the heparinized serum samples of 60 patients (25 males and 35 females) on valproic acid treatment and in different age range were selected, and the obtained results were compared.
4. Results
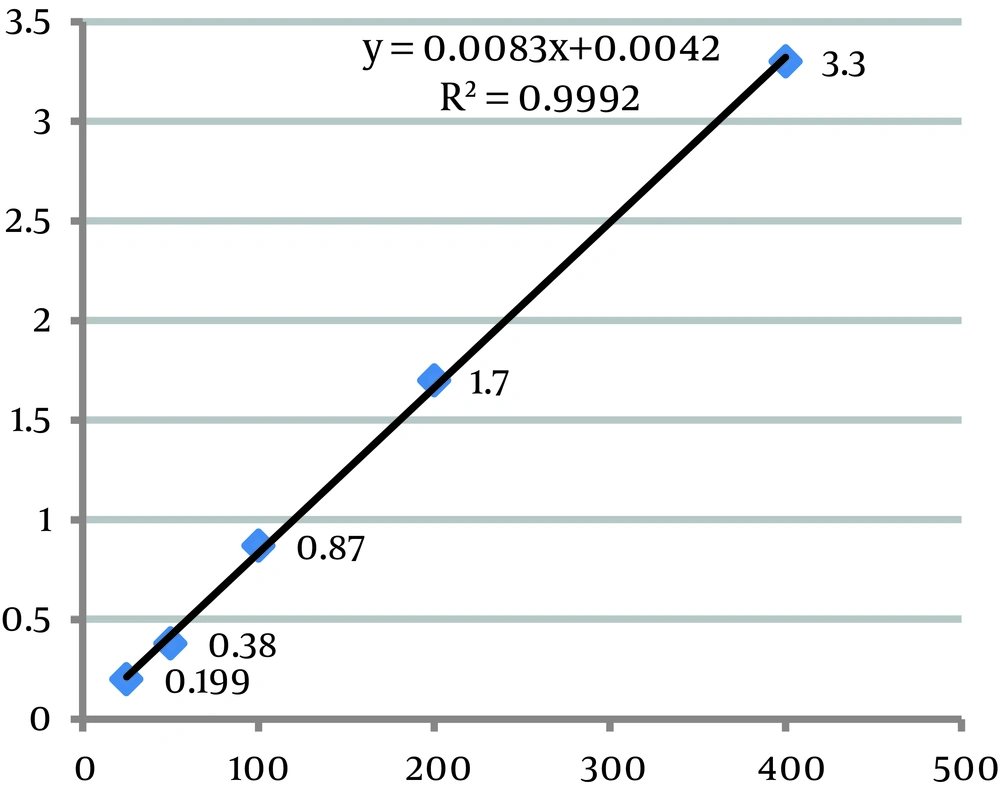
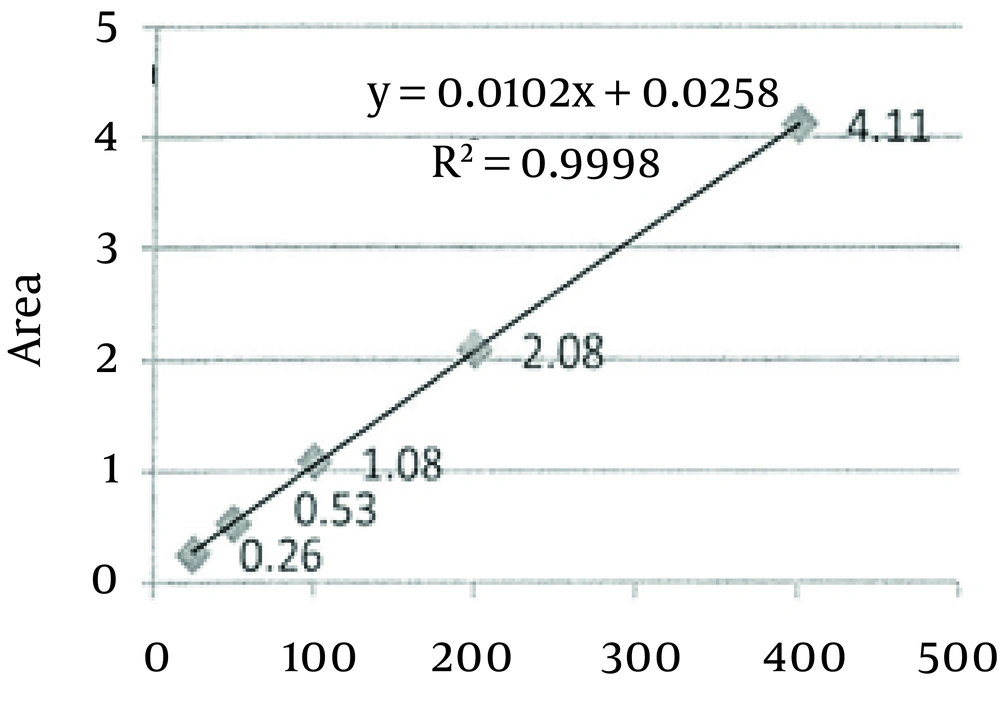
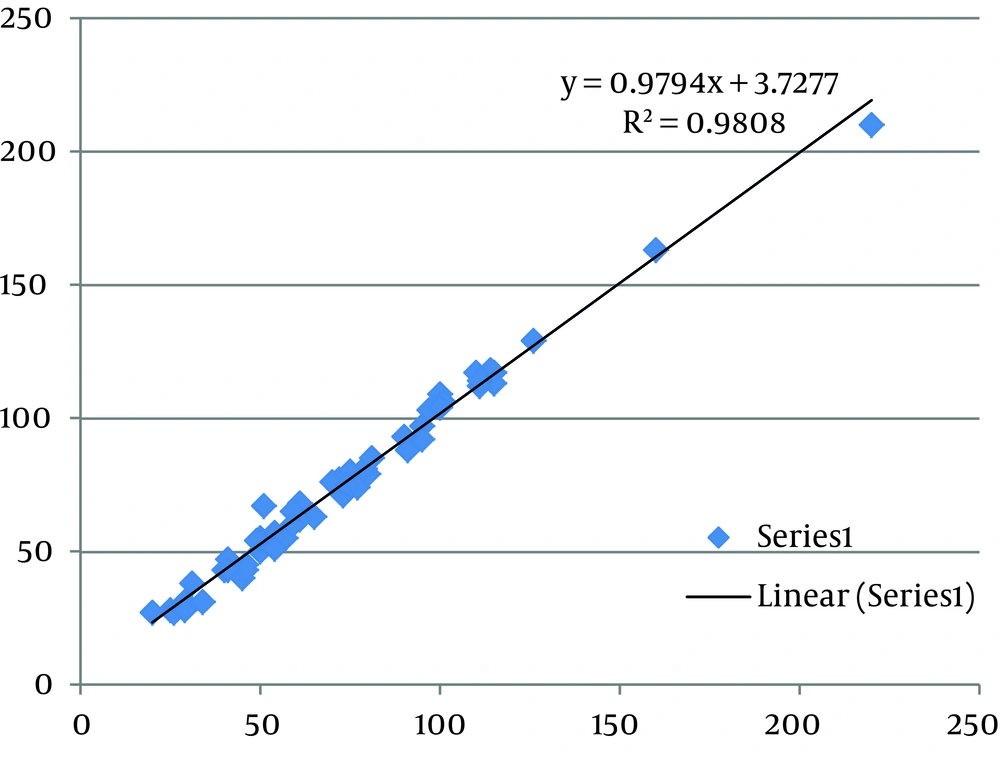
With respect to Chromsystems serum quality control material values (Table 1), obtained results for the intra-assay and inter-assay precision studies revealed a superior precision (lower CV) for GC than for HPLC in valproic acid determination (Table 2). Table 3 indicates the recovery percentage of added valproic acid in both systems. Figure 1 and 2 reveal the results obtained for the linearity studies in the linear range of 25 - 400 µg/mL for valproic acid. The limits of quantification values were 25 µg/mL for HPLC and 8 µg/mL for GC, respectively. Moreover, patients serum samples analysis correlated well in both systems (R2 = 0.98) (Figure 3).
| Target Value, µg/mL | Range, µg/mL | |
|---|---|---|
| Level I | 40.20 | 32.20 - 48.20 |
| Level II | 102 | 81.50 - 122.0 |
| level III | 150 | 120 - 180.0 |
| Intra-assay Precision, Mean ± SD, µg/mL | CV c, % | Inter-assay Precision, Mean ± SD, µg/mL | CV, % | |
|---|---|---|---|---|
| High performance liquid chromatography | ||||
| Level I | 43.29 ± 1.94 | 4.48 | 44.29 ± 2.93 | 6.61 |
| Level II | 105.50 ± 5.25 | 5.18 | 101.88 ± 6.81 | 6.69 |
| Level III | 150.35 ± 6.62 | 4.40 | 150.70 ± 10.20 | 6.77 |
| Gas chromatography | ||||
| Level I | 42.99 ± 1.66 | 3.86 | 44.09 ± 2.71 | 6.14 |
| Level II | 100.70 ± 3.97 | 3.95 | 101.48 ± 6.46 | 6.36 |
| Level III | 149.65 ± 5.97 | 3.99 | 149.61 ± 9.61 | 6.42 |
a n = 10X, 1 day.
b n = 20 days.
cAbbreviation: CV, coefficient of variation.
5. Discussion
Several different methods have been proposed for the determination of valproic acid in patients' serum samples (6-10). However, studies focusing on direct determination of valproic acid concentration in patients with epilepsy are extremely limited. Direct determination of valproic acid is difficult and, in most of the previously described GC or HPLC methods, a derivatization step after deproteinization with acetonitrile (14-16) prior to chromatographic analysis is required. These procedures are susceptible to associate with poor reproducibility (14). In addition, some of the analytic procedures may require specific detectors (15) that influence the cost of the procedures. Recently, immunological assays have become attractive for routine clinical monitoring during chronic therapy. However, in some cases they are subjected to cross reactive interference problems as well (10, 17-19).
In the present study, we developed an improved method for comparing and evaluating the determination of valproic acid using two systems (HPLC and GC). Our results in both systems agreed satisfactorily regarding linearity, correlation, and recovery studies. The obtained result for the correlation study was in agreement with previous finding (6). In another study, a minimum detectable limit of 0.5 µg/mL was reported using a derivatizing agent for the GC system (14). Our findings revealed a superior precision for the GC system; however, the limit of quantification of 8.0 µg/mL without using any derivatizing agent was reached. Furthermore, in our described GC method, the preparation step of the samples was simple and no derivatization and no complex instrument set up were required. Moreover, a higher quality of produced chromatographs and shorter run time of peaks by GC were obtained, which illustrate a good performance of the GC method for valproic acid determination among other proposed methods, especially when comparing with the HPLC system. Thus, in a laboratory analysis when making a choice for valproic acid determination either by GC or by HPLC, the proposed GC method in this study can be successfully applied for the routine quantification of valproic acid levels in patients' serum specimens.