1. Background
Synthetic dyes are widely entered into the wastewater from different industries, such as textile, tanning, pharmaceutical, leather, publishing, and printing industries. Industrial effluents are the major contaminants of the environment and, textile wastewater is a major source of environmental pollution (1-3). Dyes are categorized into azo, anthraquinone, sulfur, indigoid, triphenylmethane and phthalocyanine derivative, based on their chemical structures (4). Due to the weak performance of dyeing units and the nature of dyes, it is estimated that 50 percent of dyes are directly entered into the wastewater (5). In order to minimize the pollution risks and harmful effects of this type of material, dye effluents are carefully treated with an appropriate method before discharging the wastewater to the environment (6). So far, various techniques, such as coagulation–flocculation, oxidation, reduction, ozonation, reverse osmosis, membrane filtration, biological degradation and electrochemical, and adsorption, have been used to remove dyes (7). Due to the simple design and insensitivity to toxic substances, adsorption method is one of the most widely used and effective dye removal methods (8, 9). To date several adsorbents such as zeolites, agricultural waste, furnace bottom ash, and polymeric materials have been used for dye removal. In addition, activated carbon has been widely used as the adsorbent for dye removal (10). However, since Long and Young first reported that carbon nanotubes were more effective than activated carbon for removing dioxins, these materials as new adsorbents attracted the attention of many researchers (11). Carbon nanotubes which were divided into two groups of single-walled carbon nanotubes (SWCNTS) and multi-walled carbon nanotubes (MWCNTS) are used as a new and small-sized adsorbent with a hollowed and stratified structure (12). This new type shows a higher capacity for adsorption compared to the activated carbon and can remove dyes as well as other pollutants from wastewater (8, 13).
2. Objectives
The objectives of this study were to determine the appropriate MWCNTs dosage to adsorb RR-198 , measure the coefficients of isotherm models and identify the thermodynamic parameters such as the free energy (ΔG °), entropy (ΔS °), and enthalpy (ΔH °) changes during adsorption process at various temperatures.
3. Materials and Methods
3.1. Chemicals Materials
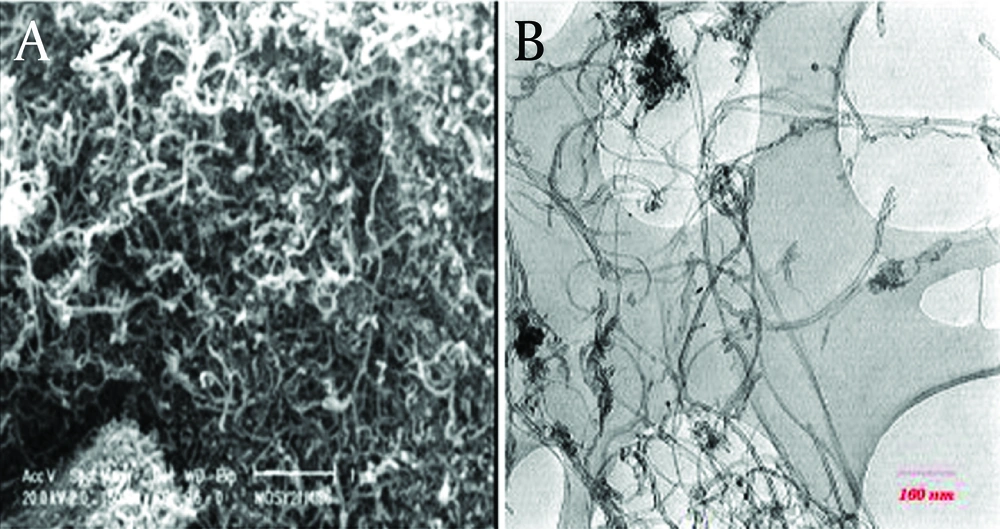
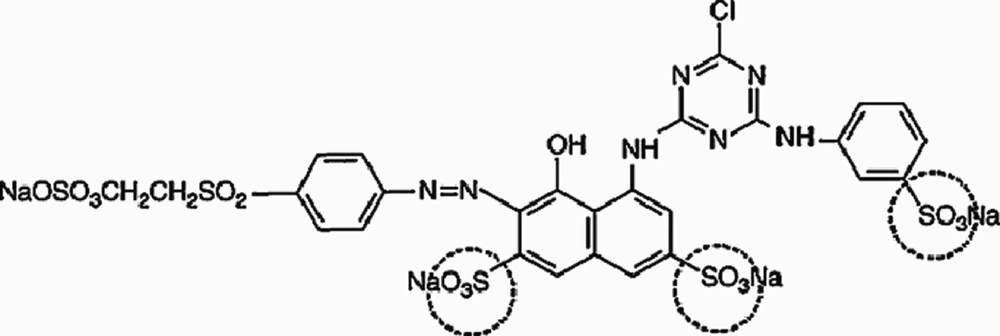
Multi-walled carbon nanotubes (MWCNTs) with above 95% purity was prepared in the Iran Petroleum Industries Research Center and was used without any further refining. According to the information obtained from the Research Center, the electrical conductivity and specific surface area of the MWCNTs used in the study were 1500 s/m and 270m2/g, respectively. Also, its length and diameter were 10 µm and 10-30 nm, respectively. The morphology of the adsorbents was characterized by scanning electron microscopy (SEM), model Tescan M-Mira in the Iran Petroleum Industries Research Center laboratory and transmission electron microscopy (TEM), and model PHILIPS EM 208 in microscopy laboratory of Nanokafa technology. Also, SEM and TEM images of the substances are presented in Figure 1. RR-198 (M.Wt968/21, Mol Formula: C27H18ClN7Na4O15S5, λmax 518 nm) used in this study was purchased from Alvan Sabet Company, Hamadan, Iran and utilized without further purification. The structure of the RR-198 azo dye is shown in Figure 2.
3.2. Adsorption Studies and Analytical Techniques
The current applied-fundamental research was conducted on synthetic wastewater in a laboratory scale. To perform the experiments, laboratory jars (250 ml) were used. In addition, HCL and NaOH were used in order to adjust pH. Then, the effect of variables, such as reaction time (5-150 min), initial dye concentration (20, 50, 100, and 200 mg/L), pH (3-10), MWCNTs dosage (100-600 mg/L), and temperature (283-303K) on dye removal was investigated. The mixing was done using a shaker (flask models rotator R430) at 150 rpm. At the end of equilibrium time, in order to separate the MWCNTS from the dye solution, the samples were centrifuged at 4000 rpm for 10 minutes and passed from 0.2 µ filter paper (14). Finally, the residual dye concentration was determined by spectrophotometer UV/ Vis (Hatch - DR5000 Germany) at the wavelength of 518 nm according to the standard method (15). Other chemicals used in this study had Merck’s laboratory purity. In this study, using one factor method at time, each parameter was separately optimized and then sample size was determined. Sample size in this study was 111.
Adsorption efficiency (%) and the amount of dye adsorbed by the adsorbent (adsorption capacity) were calculated using equations 1) and 2), respectively (16).
1) Re (%) = ((C0 – Ct)/C0) × 100
2) qt = ((C0 – Ct)V)/m
Where: Re is removal efficiency, qt, the amount of the adsorbed dye (mg/gr), C0, the initial concentration of dye in the solution (mg/L),Ct, concentration of the dye remaining in the solution (mg/L), V, volume (L) and m dose the adsorbent used (g).
3.3. Investigation of Adsorption Isotherms
To describe the mutual behavior of the adsorbent and the adsorbate and predict the adsorption capacity of the adsorbent, the isotherm equitation plays a key role as it is one of the fundamental parameters of designing the system (17). In this study, the equation of dye adsorption on MWCNTS was described using Freundlich, Langmuir and Temkin models. Equations as well as the linear forms of these isotherms are shown in Table1, (18, 19).
| Alpha Type of Isotherm | Equation | Linear Form |
|---|---|---|
| Freundlich | qe= KfCe1/n | Log qe= log Kf+ (1/n) log Ce |
| Langmuir | qe= QmKLCe/ (1 + KLCe) | Ce/ qe= (1/KLQm) + (1/Qm) Ce |
| Temkin | qe= B1Ln(KTCe) | qe= B1ln KT+ B1ln Ce |
3.4. Thermodynamic Analysis
To study the effect of temperature on the adsorption of RR-198 by MWCNTs, the thermodynamic parameters related to the adsorption process, such as the free energy (ΔG°, KJ/mol), entropy(ΔS°, KJ/mol), and enthalpy(ΔH°, KJ/mol) changes were determined by using Vant Hoff equation (20).
3) ΔH° = -RTLn (KL)
4) Ln(KL) = (ΔS° / R) – (ΔH° / RT)
5) ΔG° = ΔH° - T ΔS°
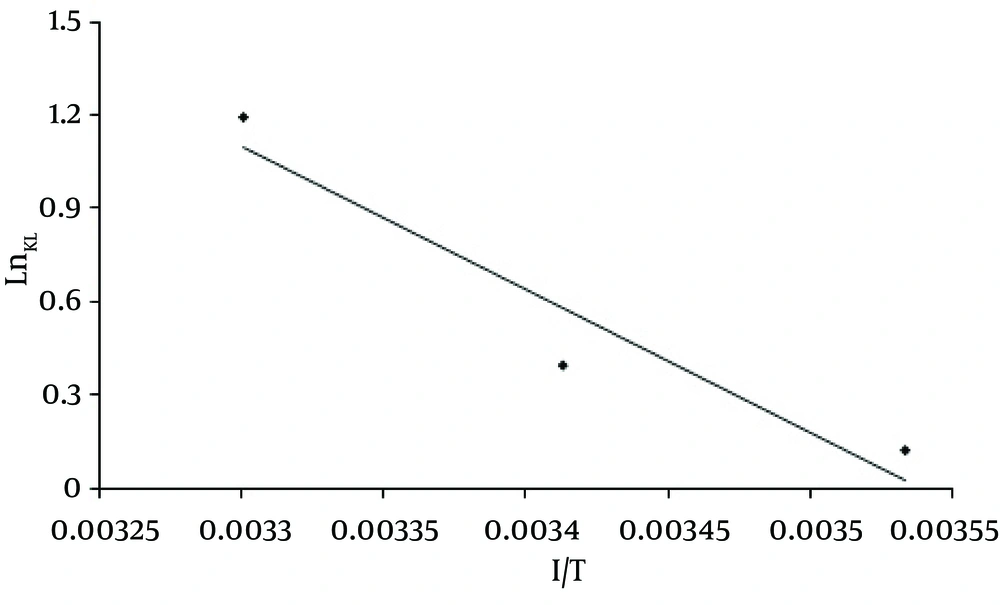
Where KL was the thermodynamic equilibrium constant (1/mol), R was the gas constant (8.314 J/mol k), T was the temperature (K), and ΔS°, ΔH° were determined from the slope of linear regression between Ln K and 1/T according to Equation 4. Also, the ΔS° values was Computed according to Equation 5.
4. Results
4.1. The Effect of pH
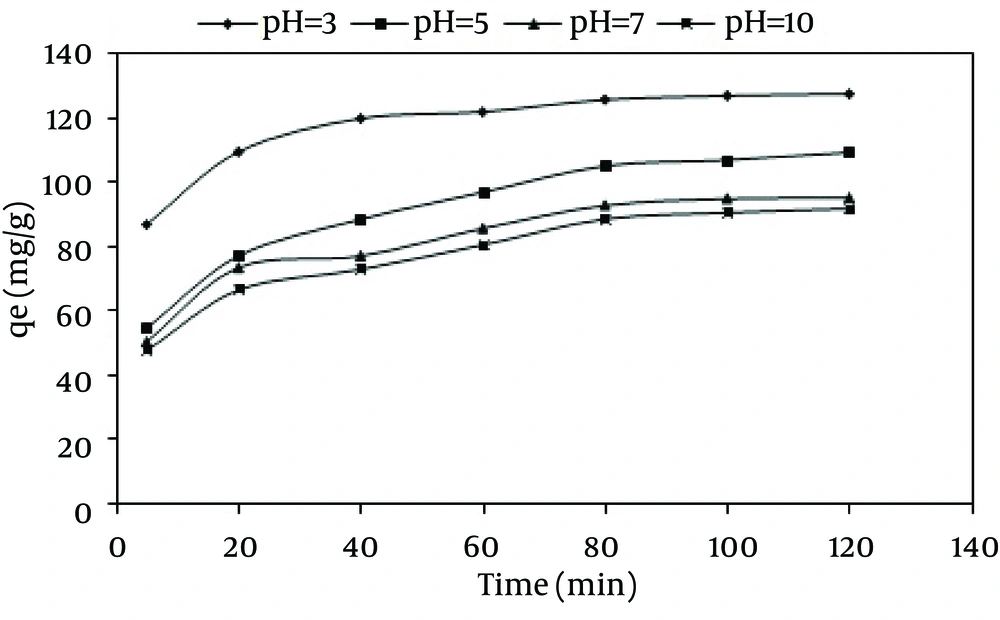
The effect of the initial pH on the adsorption process was investigated. As Figure 3 depicts, by decreasing the pH from 10 to 3 (at the dye concentration of 100 m/L and a fixed adsorbent dose (600 mg/L), the adsorption capacity increased from 91.63 to 127.233 mg/g. Besides, the maximum dye removal was achieved at pH = 3.
4.2. The Effect of Contact Time
The effect of contact time on the adsorption of RR-198 by MWCNTS in various dye concentrations is shown in Figure 4. According to this figure, by increasing the contact time, the dye adsorption capacity increased with a sharp slope during the first hour and then increased with a mild slope until reaching the equilibrium (about 120 minutes).
4.3. The Effect of the Adsorbent Dosage
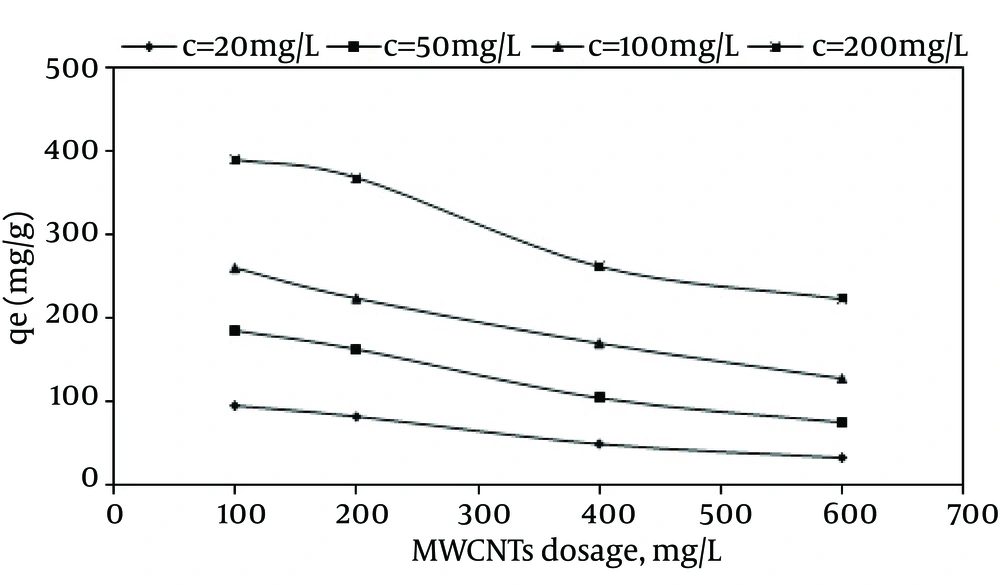
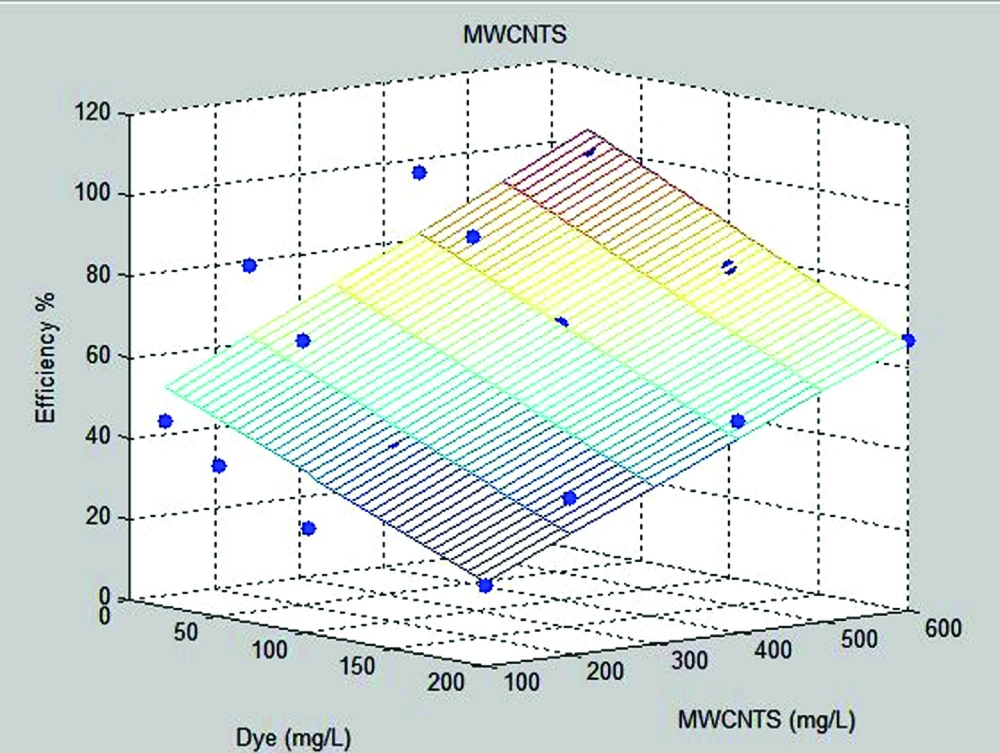
The optimal dose of MWCNTS was determined by adding different amounts of the adsorbent to various dye concentrations in pH of 3 and contact time of 120 min. The effect of MWCNTS adsorbent dose on the adsorption of RR-198 is shown in Figure 5. Based on the results, by increasing the dosage of MWCNTS from 100 to 600 mg/L at the dye concentration of 100 mg/L, the adsorption capacity was reduced from 260 mg/g to 127.95 mg/g. In addition, the maximum absorption was observed at 600 mg/L dose of carbon nanotubes. In order for better understanding of the effect of MWCNTS dose on the dye removal efficiency, the model of multivariate linear regression was drawn using MATLAB software and according to Figure 6, the best fitness was related to the linear regression coefficient with R2 > 0.9308.
4.4. The Effect of the Initial Dye Concentration
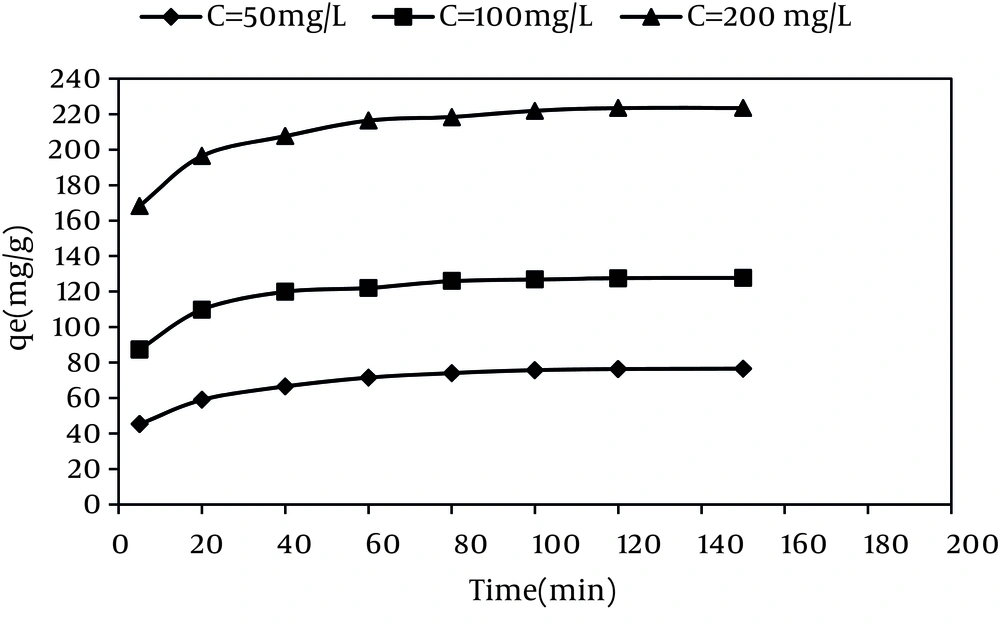
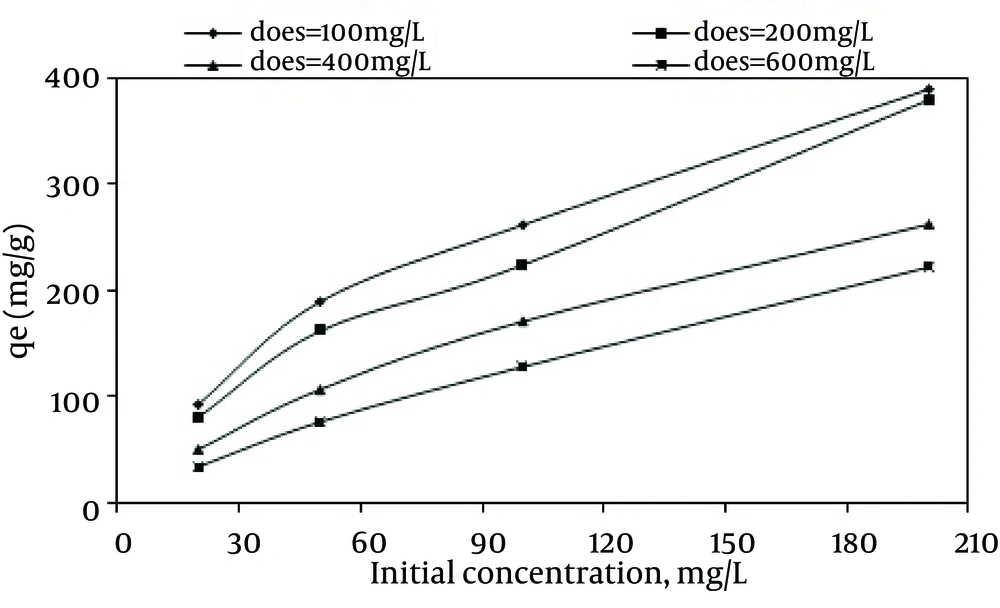
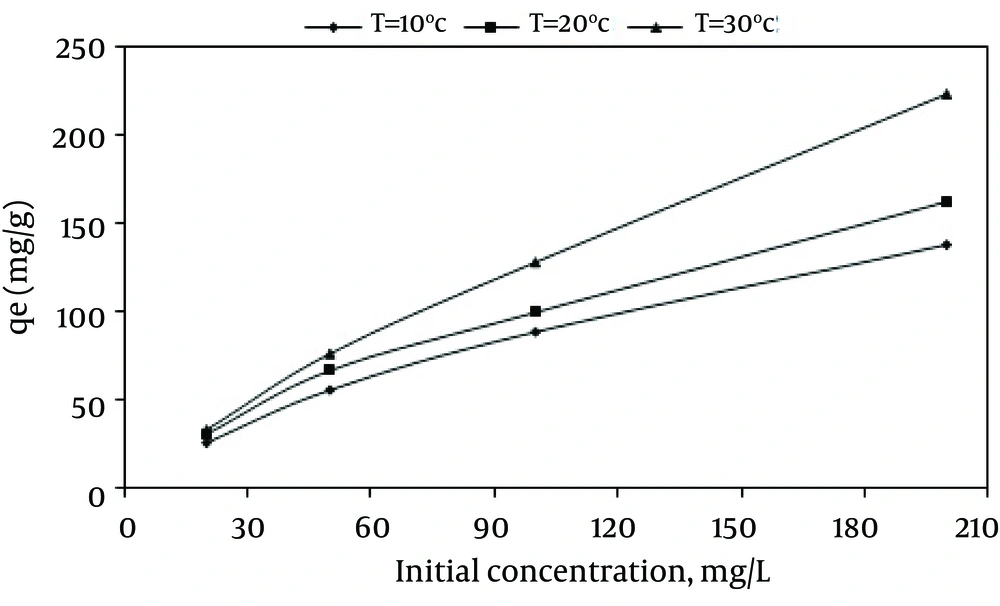
The effect of the initial dye concentration on the adsorption capacity was also studied. According to Figure 7, by increasing the initial dye concentration from 20 to 200 mg/L at the MWCNTS dose of 600 mg/L, the dye adsorption capacity increased from 33.26 mg/g to 222.88 mg/g, while the dye removal efficiency decreased from 99.62% to 66.99%.
4.5. Adsorption Isotherms
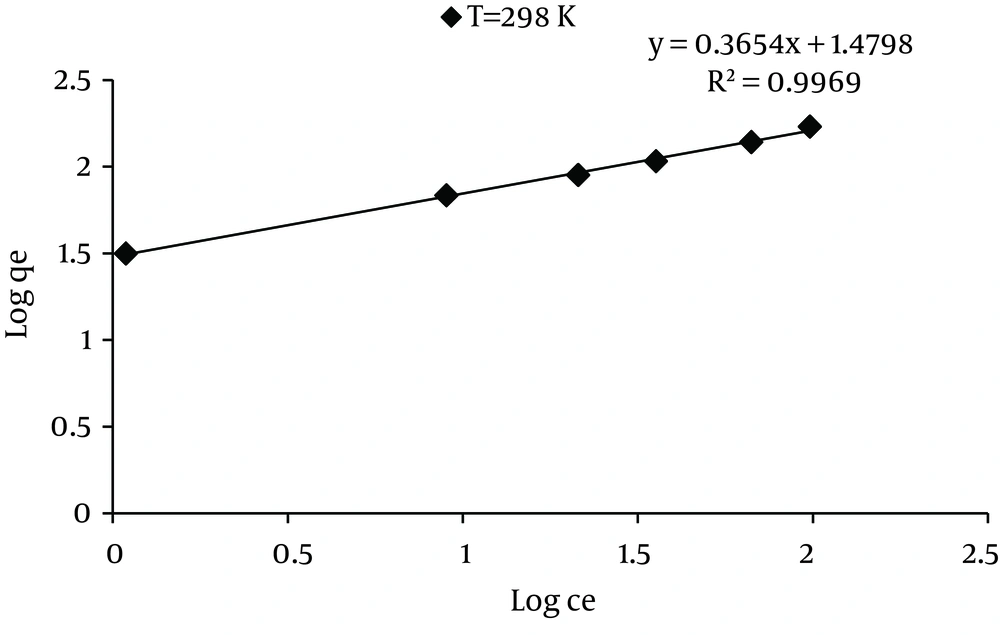
Adding the optimal dose of MWCNTS of 600 mg/L at pH = 3 and different dye concentrations, the adsorption isotherm was drawn at 298K. The study results showed that in comparison to the other isotherms, Freundlich isotherm had better correlation in dye removal by MWCNTS (R2 = 0.996 for 298K). The results of these isotherms and Adsorption equilibrium curves for dye adsorption by MWCNTS are presented in Table 2 and Figure 8.
| Alpha Type of Isotherm | Isotherm Parameters | Temperature, K |
|---|---|---|
| Freundlich | n | 2.739 |
| Kf | 30.13 | |
| R2 | 0.996 | |
| Alpha Langmuir | Qm | 200 |
| Kl | 0.056 | |
| R2 | 0.953 | |
| Temkin | kT | 1.741 |
| BT | 28.77 | |
| R2 | 0.909 |
4.6. The Effect of Temperature
The effect of temperature on dye adsorption capacity was assessed in different temperatures (283-303 ˚K) using MWCNTS dose of 600mg/L, pH = 3, and various dye concentrations. Figure 9 shows the effect of temperature on the adsorption process on MWCNTS. Based on the results, by increasing the temperature from 283 to 303 k at the dye concentration of 100 mg/L, the adsorption capacity increased from 88.3 mg/g to 127.95mg/g, The values of thermodynamic parameters at various temperatures for the dye adsorption onto MWCNTs are shown in Table 3 and Figure 10 depicts the regressions of Vant Hoff Plot for thermodynamic parameters.
| T(˚K) | (KJ/mol) ΔG ° | (KJ/mol) ΔS ° | (KJ/mol) ΔH ° |
|---|---|---|---|
| 283 | -0.06 | 134.93 | 38.119 |
| 293 | -1.41 | ||
| 303 | -2.76 |
5. Discussion
A solution's pH is an important parameter in the adsorption process. Based on the results of the present study, the maximum dye removal was observed at pH = 3. This might be due to the electrostatic reaction between negative charged anions dye and the positive charge on the surface of MWCNTS. In acidic pH levels, the surface of the carbon nanotube is charged with hydrogen ions, which leads to strong electrostatic absorption between the positive charge of the carbon surface and anions of the dye molecule and increases the absorption rate. In high pH levels, hydroxyl ions compete with the dye anions for absorption resulting in reduced dye removal (21). In the same line, the results of the study conducted by Filipkowska et al. (22) on removing the reactive dyes using Chitin showed that the maximum adsorption capacity was obtained at pH = 3. In the studies conducted by Chatterjee et al. (7) on removing congo red dye using with, chitosan hydrogel beads impregnated with carbon nanotubes, the obtained result showed that by decreasing the pH from 9 to 4, the adsorption capacity increased from 253.2 mg/g to 423.1 mg/g. Contact time: In this study, the removal rate increased by increasing the contact time. The amount of RR-198 adsorbed at equilibrium time increased from 76.33 mg/g for 50 mg/L to 127.55 mg/g for 100 mg/L and 223.41 mg/g for 200 mg/L dye concentration. This occurs due to the fact that in the early stages of absorption, a large number of blank areas are there on the surface of MWCNTS. However, as time goes by, these areas are occupied by dye molecules and the removal efficiency remains constant after the equilibrium. At this stage, the repulsive force of the dye molecules adsorbed on the adsorbent surface prevents the absorption of new molecules. Ai L et al. (23) conducted a study on methylene blue dye removal using MWCNTs charged with magnetic particles. The study results showed that the removal efficiency increased with the increase in time and then followed a steady slope and reached equilibrium after 120 min.
Adsorbent dose: According to Figure 5 and 6, as the adsorbent dose increased, dye removal efficiency increased, as well. This can be due to the increased in the adsorbent specific surface area for absorption, as well as the increase in the amount of the adsorbed dye, Also, with an increase in the adsorbent MWCNTs dose, the adsorption sites and the surface are extended and more activated sites will be available (9, 24). By increasing the amount of the adsorbent reduction of the absorption capacity can be attributed to the inter-particle reactions, such as density, which reduce the surface area of the adsorbent and increase the distance between the absorbent and the adsorbate, However, high multi-wall nanotubes dosage may increase the viscosity and inhibit the diffusion of dye molecules to the surface of the multi-wall carbon nanotubes (25). WU (9) also conducted a study on reactive dye removal using carbon nanotubes and showed that by increasing the adsorbent dose to above 0.25 g/L, the adsorption capacity declined. Also, in a study conducted by Kuo et al. (6) on direct dyes removal using carbon nanotubes, it was found that by increasing CNTs dosage from 0.33 to 0.67 g/L, the adsorption capacity decreased from 12.7 to 11.1 mg/g.
Initial concentration: The findings of the current study showed that as the dye concentration increased, the adsorption capacity increased, as well. This might be due to an increase in the driving force of the concentration gradient with the increase in the initial concentration (21). Zhao et al. (26) conducted a study on the removal of methyl orange dye and found that by increasing the initial dye concentration from 8 mg/L to 20 mg/L, the adsorption capacity increased from 25.3 mg/g to 44.7mg/g for Multi walled carbon nanotubes.
As Table 2 depicts, Freundlich isotherm model had a better correlation compared to the other isotherms. Also, the values of KF and n from this isotherm model are listed in Table 2. It was observed that as the temperature increased, the value of KF increased. Higher value for KF indicates higher affinity for adsorbate. Besides, by increasing the dye equilibrium concentration, the adsorption equilibrium capacity increased and maximum adsorption capacity of MWCNTs was obtained as 161.73 for 298K, respectively. In general, values of n in the range of 1–10 show that adsorbate was favorably adsorbed onto adsorbent (12). The values of n 2.73, at 298K is greater than 1, so they represent the favorable removal conditions. In the studies conducted by Qu et al. (8), the obtained result showed that two dyes producing from the removal of carbon nanotube embedded with Fe2O3 particles at R2 = 0.986 for Neutral Red and R2 = 0.983 for Methylene Blue, respectively, and followed the Freundlich isotherm.
Temperature: The findings of the current study showed that as the temperature increased, the absorption capacity increased, as well. This indicated that , at high temperature, the mobility and diffusion rate of dye molecules increased, while the solution viscosity decreased, which is due to the existence of available empty surfaces during the early stages of adsorption .As a result, the adsorption capacity of the system will be higher (9, 27).
Thermodynamic analysis: As Table 3 depicts, as the temperature increased from 283 to 303 K, the value of ΔG ° decreased from -0.06 to -2.76 KJ/mol, Negative values of ΔG ° indicate that the sorption process is more spontaneous at higher temperatures. The value of enthalpy change ΔH ° shows the exothermic or endothermic of the adsorption process. Kara et al. (28) suggested that the ΔH ° of physisorption is smaller than 40 KJ/ mol. On the other hand, the positive value of ΔH ° (38.119 KJ/mol) indicates that the adsorption process of RR-198 onto MWCNTs is endothermic and physisorption. The positive value of ΔS ° (134.93 KJ/mol) also, shows the increase in the degrees of freedom at the solid-liquid interface during RR-198 adsorption on MWCNTs (13, 19, 29). The results of the study conducted by Duan (30) on removing methyl violet dye also showed that by increasing the temperature from 288 to 308 °K, the adsorption capacity increased from 88.50 to 90.91 mg/g. In the studies conducted by Konicki et al. (7) Negative values of ΔG ° (-5.59 to -7.90 KJ/mol) and the positive value of ΔH ° (12.6 KJ/ mol) showed that, the adsorption of Direct Red 23 onto MMWCNTs-ICN was spontaneous and endothermic also, (Table 4).
| Nomenclature | |
|---|---|
| Kf | freundlich isotherm constants (L/g) |
| KL | langmuir isotherm constants (L/mg) |
| n | adsorption intensity |
| qe | equilibrium adsorbent concentration on adsorbent (mg/g) |
| qecal | calculated values of qe(mg/g) |
| Qm | maximum monolayer capacity (mg/g) |
| qt | adsorbed metal concentration at time t (mg/g) |
| R2 | correlation coefficients |
| Ce | equilibrium concentration in solution (mg/L) |
The results of this study showed that, the time for reaching equilibrium for dye adsorption on MWCNTS adsorbent was equal to 120 minutes. Although all the parameters under study were significant and effective, the solution's pH played more important role in the adsorption process of RR-198 on MWCNTs. And acidic pH was favorable for the adsorption of RR-198. Moreover, the thermodynamic parameters (ΔH °, ΔG °, and ΔS °) indicating that the adsorption of RR-198 onto MWCNTS was spontaneous and endothermic. Also, using MWCNTS as an effective adsorbent is recommended for dye removal from textile industries.