1. Background
The disposal of a great quantity of organic wastes produced by the municipal, agricultural and agroindus trial activities causes energetic, economic and environmental problems (1); but, municipal solid wastes (MSW), for their high content in organic matter and mineral elements, could be used to restore soil fertility. Composting process is a useful method of producing a stabilized material that can be used as a source of nutrients and soil conditioner in fields (2). Composting is an aerobic and exothermic process used in treating biodegradable waste for use as a soil conditioner and fertilizer worldwide. It is also the most environmentally friendly method of waste treatment comparative to other known methods such as incineration, landfill and anaerobic digestion (3, 4). It is a process in which microorganisms degrade organic waste. As a microbial process, it is influenced by all factors that affect microbial life: temperature, pH, moisture, air (oxygen), and nutrients. Composting process consists of 3 - 4 weeks of biodegradation in the active phase, followed by several months of maturation in the curing phase (5).
Composting results in a decrease in mass and volume of organic wastes, a reduction in land filling problems, and biodegradation of toxic compounds and other organic contaminants (6, 7). The resulting product can act as a soil conditioner that may increase soil fertility and structure, porosity, organic matter levels, water holding capacity, cation exchange capacity, as well as contribute to the suppression of plant pathogens (8-10). In addition to these benefits, compost products can replace the use of non-renewable organic products (11) and also composts can be used for the suppression of diseases caused by soil-borne plant pathogens (12).
Compost stability and compost maturity are the two terms often used to describe the rate of decomposition and transformation of the organic matter in compost. Compost ‘stability’ and ‘maturity’ are not synonymous. Compost stability refers to the compost in which the rate of energy release due to microbial degradation of the organic matter equals the rate of energy loss to the environment. Under these conditions, the temperature of the compost remains constant and equals that of the ambient (13). Compost maturity is an important factor affecting the successful application of composts for agricultural purposes (14, 15) and their general marketability (16). In addition, maturity is a term used to indicate the level of phytotoxic substances in composts and compost suitability for plant growth (17-19). In fact, stability is a word associated with the resistance of various organic matters of a product against extensive degradation or toward major microbiological activity and maturity describes the ability of a product to be used efficiently in agriculture and is related to the growth of plants and to phytotoxicity aspects. Generally, both criteria should be somehow correlated, since phytotoxic materials are products of the microbial activity of unstable organic matter.
Application of unstable or immature compost may inhibit seed germination, reduce plant growth, and damage crops by competing for their oxygen or causing phytotoxicity to plants due to insufficient biodegradation of organic matter (18, 20, 21). Therefore, only the use of mature compost can guarantee its employment in agriculture without any damaging effect to both soil and plant.
At present time, a number of criteria and parameters have been proposed for testing compost maturity. Maturity parameters are based on different properties: physical, chemical and biological, including microbial activity (3). Some of the methods that have been used to measure maturity include C/N ratio, changes in nitrogen species, pH, electrical conductivity, cation exchange capacity, organic chemical constituents, reactive carbon, humification parameters, optical density, temperature, color, odor, structure, specific gravity, plant assays, respiration, microbial population changes, enzyme activity germination tests, and calorimetric and spectroscopic methods (10, 22-26).
Unfortunately, all of these tests are only suitable for specific types of compost and are inadequate parameters for assessing compost maturity.
2. Objectives
The main objective of this study was to evaluate a variety of maturity indices and heavy metals content in compost during the composting process and to suggest an appropriate parameter for assessing composted municipal solid wastes in a biocompost factory.
3. Materials and Methods
3.1. Description of Semi-Industrial Plant
The study was carried out in an experimental composting plant in Zahedan city, Iran. The capacity of this plant was about two tons per hour. The waste collected from various sites of the city of Zahedan goes successively to the following steps: manual and mechanical sorting treatment, iron removal, and rotating drum sieve. The compost material (mean granule size 4.0 cm) comes out from the drum sieve and is transported to the composting site to start biological transformation. The dimensions of the windrow studied were 1.5 m × 2 m × 7 m (height × width × length). The composting materials were mechanically turned twice a week for six weeks and without any mixing for the remaining period. The windrows were turned using a front-end truck loader.
3.2. Sampling of the Compost Mass for Physicochemical and Microbiological Analyses and Germination Index Determination
Changes in the chemical characteristics, stability and maturity of the composting mixture with time were studied by collecting and analyzing the samples at different times. Compost masses were sampled at the top, middle and bottom locations of pilot (in depths 20, 50 and 100 cm of the pile) weekly for laboratory study on moisture, pH and microbial analysis. However, temperature measurements of compost masses were done in situ. Temperature (daily) and water content (weekly) of the composts were measured throughout the process (thermometric probes of 1 m length allowed the daily control of temperature outside and inside the windrow). The samples taken were bulked to obtain a representative sample, packed with ice cubes in an ice chest, and transported each and every week to the laboratory. The samples were kept in freezer at 4°C for a day before the microbial and chemical analyses were performed.
The moisture content of the fresh samples was determined as weight loss upon drying at 105ºC in an oven for 24 hours. The pH and electrical conductivity (EC) of the sample were measured using a 1:6 (w/v, compost/water ratio) sample to deionized water extract. Then, the mixture was allowed equilibrating for 30 minutes with occasional stirring with a glass rod. The resulting solution was filtered through a Whatman No. 40 filter paper and used for direct measurement using a pH meter model E520 (Metrohm Herisau, Switzerland) and a Jenway conductivity meter (Model 4200).
The percentage of organic matter was determined as sample weight loss (previously oven-dried at 105ºC) upon ashing at 550°C for four hours in a muffle furnace. The total Kjeldahl nitrogen (TKN) content was quantified on 50 mg of dried ground sample by mineralization with a strong acid medium (98% H2SO4), followed by steam distillation and titrimetric determination (macro-Kjeldahl distillation method). Total carbon content of the samples was determined through combustion in oven at 750°C for two hours.
Dried and ground samples were digested by HNO3 and HClO4 (5:1 ratio, v/v) to determine heavy metals concentrations in the compost. A 1.00 ± 0.01 g sample was digested after adding 36 mL of acid mixture solution. Zn, Cu, Cr, Ni, Cd and Pb were determined in the resulting solution by atomic absorption spectrometry.
Plant assays were performed using a direct-seed test of garden cress in soil/compost mixtures (26). For determination of the germination index, two sterile Petri dishes were taken and labeled to represent the systems under study. Layers of number 2 filter paper pad were fitted into each Petri dish and then wetted with 5 mL of 1:10 compost aqueous extract. A third Petri dish was taken and fitted with two layers of filter paper and distilled water was used as the medium of wetting the filter paper pads to serve as control. Twenty cress seeds were placed in each Petri dish and incubated in the dark at 22 ± 2°C for five days (with 10 hours of light per day). The seeds were examined for germination, where germination was defined as any protrusion through the seed coat. The percentage of normalized cress seed germination, GN (%), was calculated for each sample using the following equation:
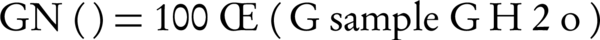
Where GSample = number of cress seeds germinated in the assay and G H20 = mean number of cress seeds germinated in the water control for the assay.
3.3. Microbiological Analysis
The presence of Salmonella spp. and the enumeration of indicator microorganisms (coliforms and fecal coliforms) were determined using standard bacteriological methods (27). All the results reported in the text were the means of determinations made on three replicates and reported on a dry-weight basis.
4. Results
4.1. Monitoring of the Composting Mixture General Parameters
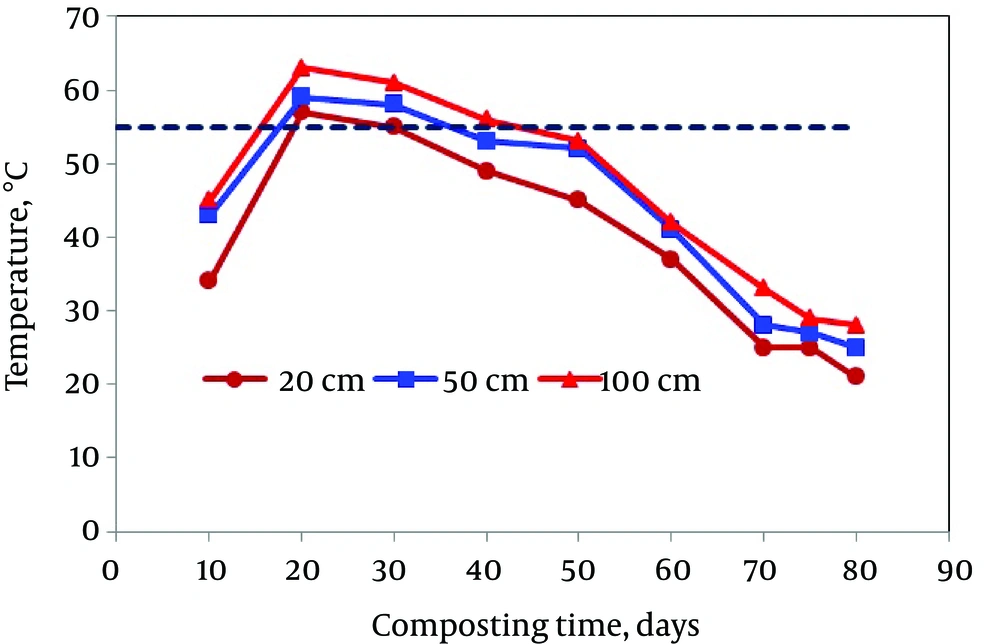
Measurements of temperature within the composting matrix during the active and curing phases gave an adequate real-time indication of the establishment of ideal conditions that support microbial degradation. Figure 1 shows a time-temperature curve during composting of municipal solid wastes. As shown in Figure 1, the temperature of the matrix increased speedily to 34 - 63°C (during the active phase) and continued during the whole active phase. Maximum temperatures were obtained after 20 days and the matrix temperature was greater than 55°C for more than three days (> 20 days at depth of 100 cm), the minimum requirements for a proper disinfection of waste materials from animal and plant pathogens as well as for weed seed suppression. The establishments of these thermophilic conditions were due to the self-heating of organic matter as a result of microbial respiration and were indicative of an adequate waste composition capable of supporting aerobic microbial degradation. Compost producers should closely monitor temperature and duration of the compost pile to minimize the risk of human and plant pathogens in the wastes and to destroy the weed seeds. However, temperature does not appear to be a reliable standard in evaluating maturity because a decrease in temperature also occurs if the pile becomes too wet or too dry (28).
Measurement of temperature at different depths during composting (Figure 1) was essential to ensure that the entire mass met temperature requirements, since the outer extremities usually have lower temperatures than the center. Increasing temperatures were obtained with depth; so, the maximum temperatures were obtained at depth of 100 cm (63°C). Additionally, as it can be seen in Figure 1, temperature of the compost mixture at different depths gradually reduced after the active phase and finally reached to the temperature of environment. The decrease in the pile temperature was attributed to the depletion of easily degradable organic materials (29). Similar findings were reported by Hassen et al. (30) and Liu et al. (31).
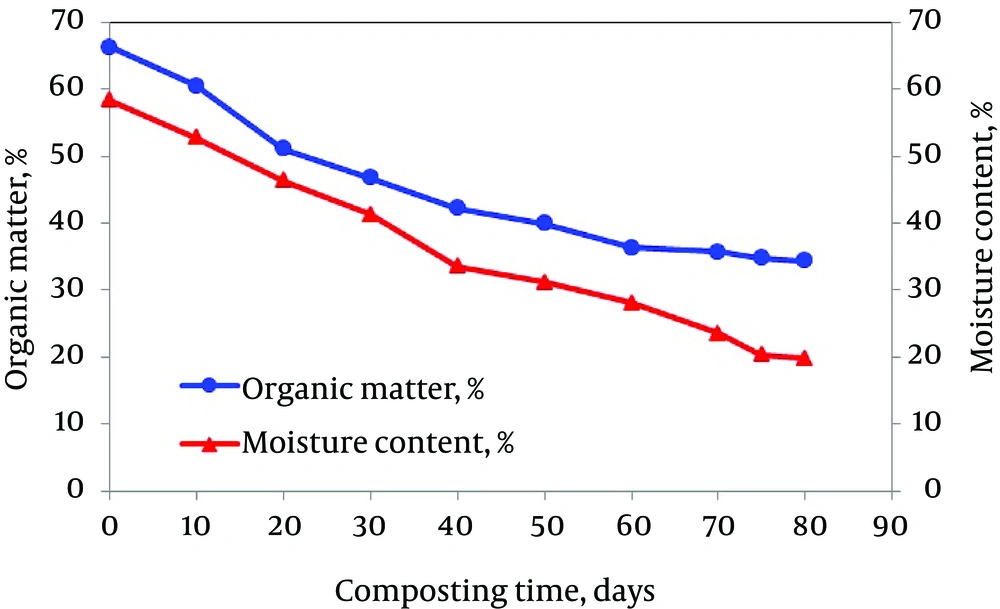
During the active phase of the composting process, the moisture content decreased from 58.3% to 38.8% (Figure 2) with most of the reduction occurring during the first 30 days, primarily due to matrix turning, positive aeration, and temperature elevation. In addition, the moisture content reached to 19.8% at the end of the composting period (after 80 days). Moisture content during the active phase of composting is a function of temperature and rate of aeration. However, the optimum moisture content is between 50% and 60% (28). Moisture content was always found to be close to 45% - 50% for most part of the active phase and the final decrease towards the end allowed efficient material handling thereafter. Furthermore, as seen in Figure 2, the percentage of organic matter of the compost mixture decreased from 66.25% at the beginning of composting to 34.38% at the end of process (31.87% reduction) (after 80 days). Maximum reduction in organic matter (24.03%) was observed during the first 40 days of composting (active phase) with high microbial activity and high temperature in the mixture of compost.
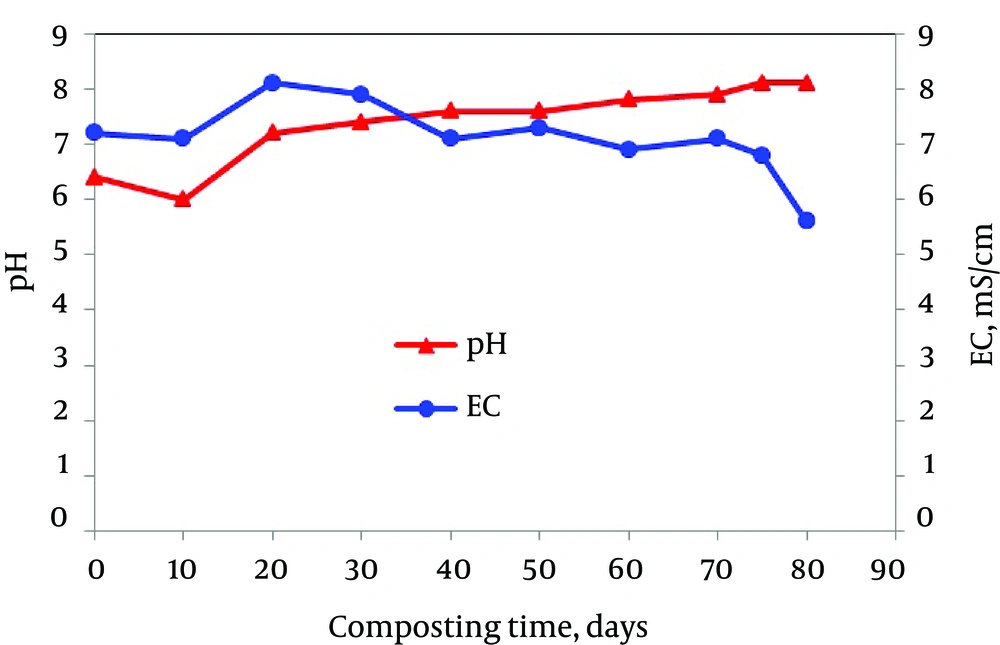
In addition, Figure 3 shows pH variation and electrical conductivity of the aqueous extract of the composting mixture with time of composting. The drop in pH during the very early stages of composting is typically due to anaerobic conditions that are established in the waste materials prior to the commencement of the composting process, resulting in the formation of organic acids. As aerobic conditions are provided through mechanical aeration and turning of the composting mass, an increase in pH is observed as these organic acids are degraded. During the process, the mineralization of proteins, amino acids and peptides leads to the release of ammonium or volatile ammonia and also contributes to the increase of pH. The pH increase to alkaline ranges is attributed to the consumption of protons during the decomposition of volatile fatty acids, the generation of CO2 and the mineralization of organic nitrogen. Alkaline pH is desirable since it limits the availability of heavy metals; on the other hand, it can induce a micronutrient deficiency (31-33).
The results of this study are consistent with the findings of Said-Pullicino et al. (32) and Liu et al. (31) who reported similar results using municipal solid waste compost and composting of dairy manure with rice chaff. Avnimelech (33) also reported similar results and further explained that pH and electrical conductivity (EC) changes were caused by decomposition of organic acids, suggesting that simple parameters such as pH and EC might be good indicators of compost stability.
EC also affects the quality of composts in a large way because it reflects their salinity and suitability for crop growth. Soluble salts, as estimated by electrical conductivity determination (Figure 3), were typically fairly high throughout the composting process, with values in the range of 5.6 - 8.1 ms/cm. The EC value of mixture reduced from 7.2 ms/cm at the beginning of the composting process to 5.6 ms/cm at the end of the process. The decrease of EC in the composting process is the direct consequence of the increased concentration of nutrients, such as nitrate and nitrite. Nevertheless, the relative uniformity of conductivity measurements across sampling dates was expected, since leaching from the composting mixture did not occur during the active composting or curing. A similar finding was reported by Said-Pullicino et al. on composting of municipal solid waste. On the other hand, Said-Pullicino et al. measured high EC values (5.0 and 7.8 ds/m) during the composting of biowastes, pruning wastes and leaves; however, the compost product was not phytotoxic in germination tests. However, others suggested that compost with high EC content could present a phytotoxic behavior affecting seed growth when used in large amounts (32).
4.2. Monitoring of the Chemical Parameters of Composting Mixture
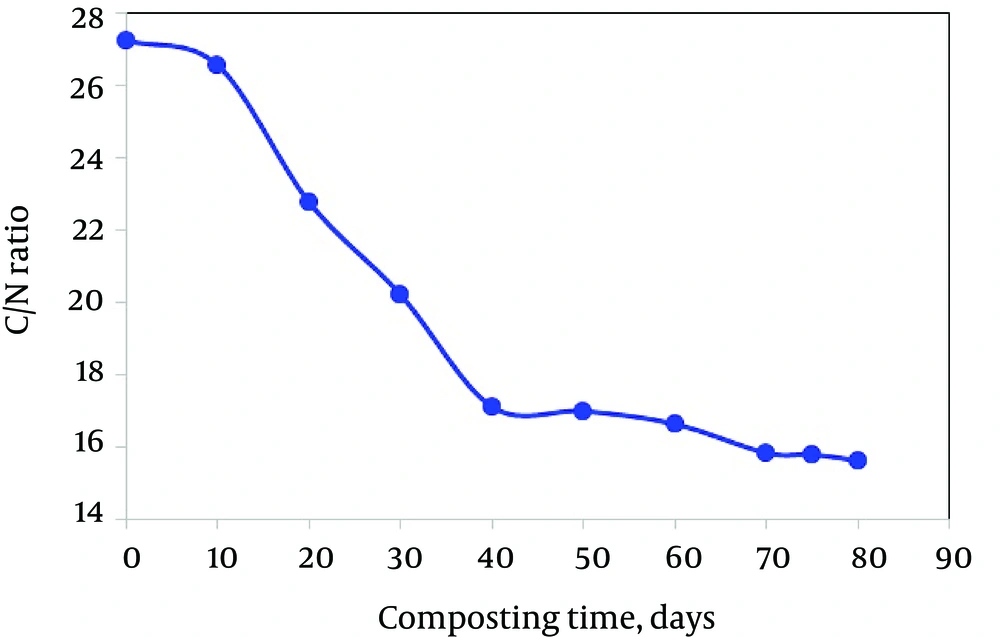
The C/N ratio has frequently been used to describe organic waste decomposition and it is widely accepted that a high substrate C/N ratio implies a low mineralization rate due to N deficiency. Furthermore, composts with high C/N ratio can cause nitrogen immobilization upon amendment to soil and those with low C/N ratio can cause ammonium toxicity (28). Researchers have suggested various ideal C/N ratios ranging from 12 to 25 (34). The C/N ratio in the compost bulk decreases during composting regardless of the compost technique employed. On the other hand, when an organic waste is composted, the C/N ratio generally decreases throughout the composting process due to the carbon losses as CO2 and then stabilizes in the range of 10 - 15 (35). A ratio of 10 - 15 is considered stable; however, it may level off much before the compost stabilizes (35, 36) and the final ratio depends on the source materials and on the method of N measurement (37). According to Rosen et al. (38), a C/N ratio between 15 and 20 was ideal for ready-to-use compost. However, Inbar et al. cautioned that the C/N ratio of compost was only one parameter by which maturity should have been gauged and specific chemical analyses were equally important (14).
In our experiment, the C/N ratio significantly decreased from 27.26 to 15.64 at the end of the composting period (Figure 4) and was similar to results in previous studies (39). Data were variable and did not have a consistency during the transition period from active to stable composting time. Maximum reduction in the C/N ratio occurred during the active phase (up to 40 days) and then the rate of reduction slowly continued. Indeed, the C/N ratio decreased during the composting process due to the transformation of carbon into carbon dioxide followed by a lower decrease in the concentration of organic acids (40).
If high C/N compost is added to soil, soil microorganisms compete with crops for available nitrogen, thereby reducing growth. Hence, with respect to the C/N ratio (15.64), the final compost of this study obtained after 80 days can be used safely in agriculture. In a similar study, Hachicha and Ghoul showed that the maturity of the municipal solid waste compost was reached only after 70 to 75 days according to stabilization of the C/N value (41). This difference in duration could be attributed essentially to the nature of the substrate and experimental conditions. Therefore, the C/N ratio alone is not a sufficient criterion to determine the compost maturity as was found in the case of sewage sludge compost which sometimes presented C/N values lower than 15, despite the instability of the product due to the high N-richness of the waste (42).
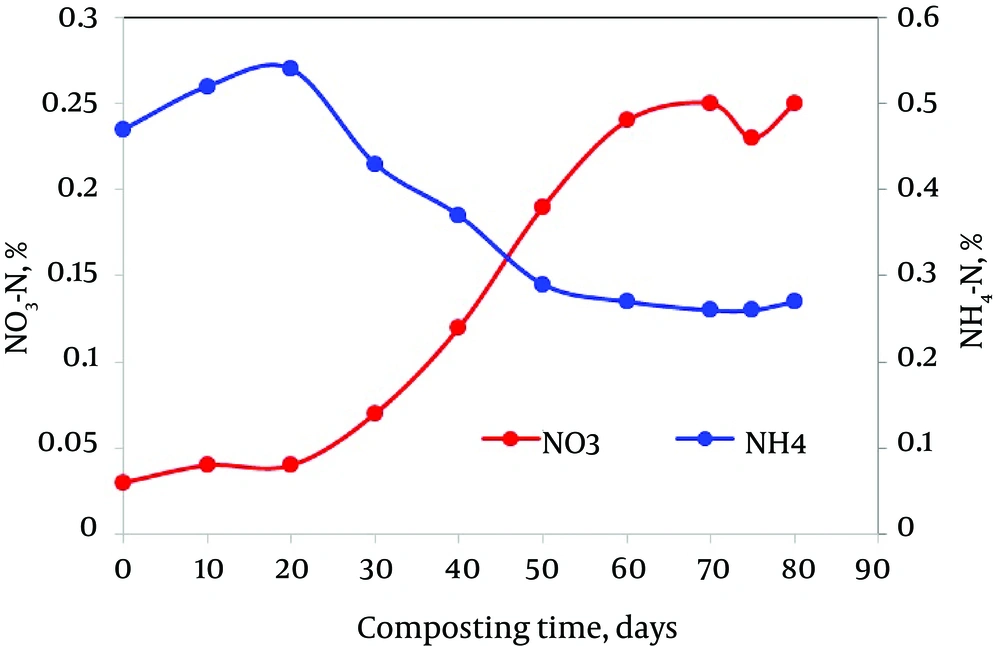
Mineral N concentration was highly variable, but an overall pattern is evident (Figure 5). Extracts of fresh compost showed high NH4-N and very low NO3-N concentration during the early stages of composting. As composting proceeded, NH4-N levels generally declined, with NO3-N increasing substantially in late samples. On the other hand, a drastic increase in nitrate concentration was observed during the maturation phase. Chikae et al. (22) reported that the NO3-N concentration increased with the reduction of NH4-N. This result could be explained by the report that bacteria responsible for nitrification were strongly inhibited by temperature greater than 40°C (43); so, nitrate concentration did not change greatly during the active composting stage. Similar findings were reported by Qian et al. on co-composting of livestock manure with rice straw (44).
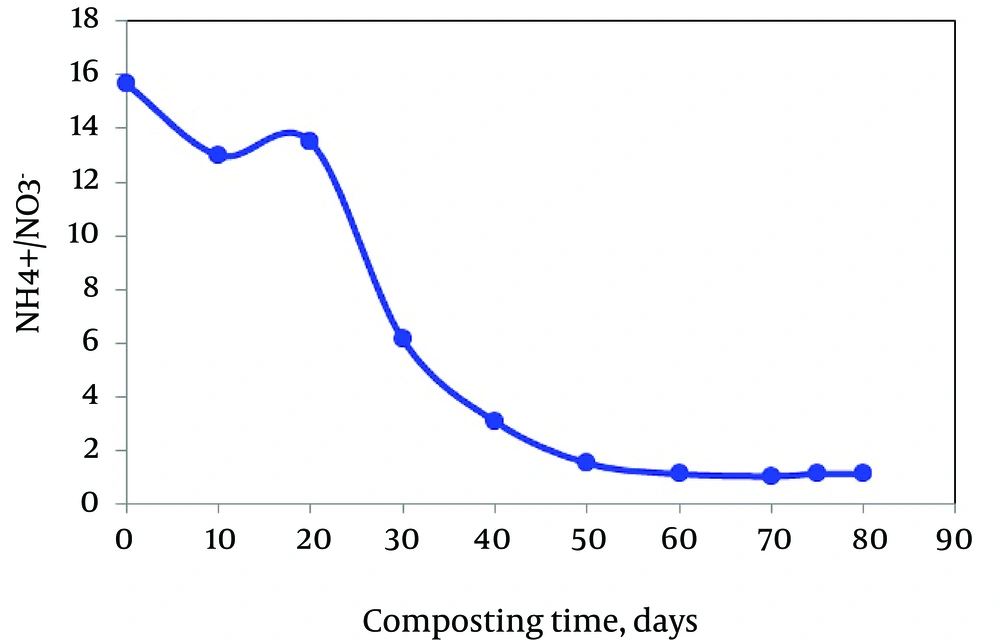
In addition, the ratio of NH4-N to NO3-N in the water extract has been suggested as an index of maturity. However, the final value of NO3- reached depends on the source material and neither any particular level of NO3- nor its ratio to NH4 can be relied upon as an indicator of compost biomaturity (45). It should also be noted that the increase in NO3- is gradual over a lengthy period of time; thus, the determination of the point at which the increase begins is difficult. As seen in Figure 6, the NH4+-to-NO3 ratio greatly decreased during the active phase due to the high levels of NO3 detected at this stage. As mentioned above, the NH4+-to-NO3 ratio has also been used to estimate the compost stability (45), giving a limit value of 0.16 for stable composts. In our study, this value was exceeded during the maturation phase (1.08) due to the low content of NO3 detected during this stage (Figure 6). As mineral nitrogen forms changed irregularly with the composting time, they cannot be reliable indicators. These results are similar to the findings of Eggen and Vethe (46) regarding ammonia-to-nitrate relation in maturing compost. A similar finding was also reported by Gomez-Brandon et al. (47).
4.3. Monitoring of Microbial Parameters and Germination Index
Pathogenic organisms, present in various organic materials, are a potential public health threat to site operators and compost users. Pathogens belong to four main groups: bacteria, viruses, parasites, and fungi. In composting, heat is the primary factor in pathogen inactivation. Thermophilic temperatures must be reached and maintained for adequate time to inactivate pathogens effectively. At the present study, microbial parameters including Salmonella and total and fecal coliforms were monitored during the composting process. The presence of coliform is often used as an indicator of the overall sanitary quality of soil and water environments. Use of an indicator such as coliforms, as opposed to the actual disease-causing organisms, is advantageous as the indicators generally occur at higher frequencies than the pathogens and are similar and safer to detect.
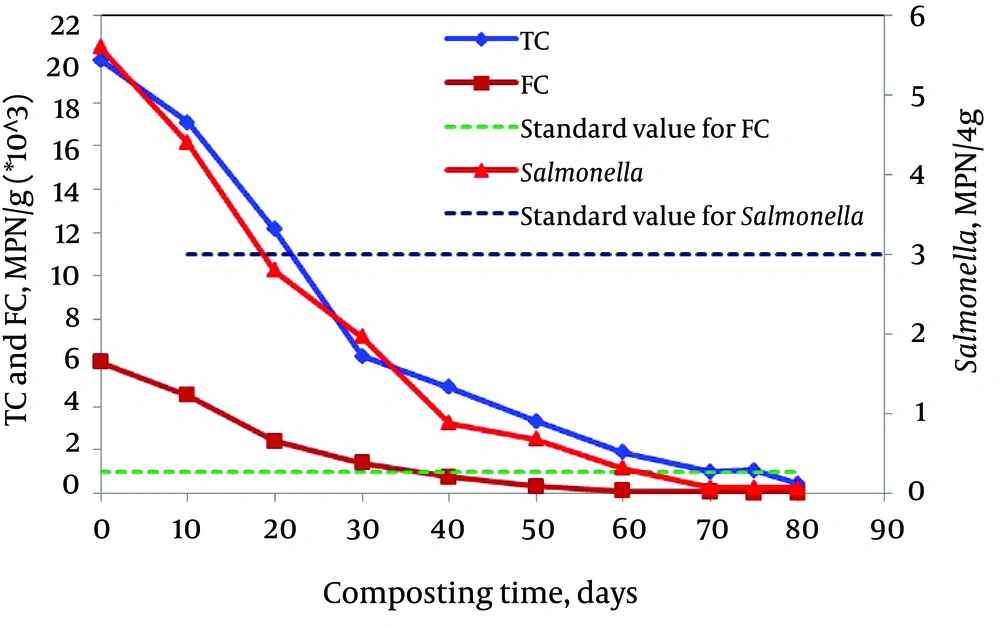
As shown in Figure 7, the average number of total and fecal coliforms were 19952 and 6045 most probable number (MPN)/g of dry solids at the beginning of the composting process and decreased to 4879 and 749 MPN/g, respectively during the active phase (after 40 days) and covered the regulation limits for fecal coliforms. This decrease was presumably the result of the high temperature (more than 60°C) and of the unfavorable conditions established during the thermophilic phase (30). In addition, at the end of the composting time, the average number of total coliforms reached to 442 MPN/g of dry solids and fecal coliforms were not detectable. A similar finding was reported by Bazrafshan et al. (48).
The presence of Salmonella is considered as the major and specific problem of the hygienic quality of compost (49). This was probably because these bacteria are ubiquitous and have a capacity for very fast growth. The United States Environmental Protection Agency (US-EPA) imposes for Salmonella a rate of < 3 MPN/4 g total solids on a dry weight basis for safe application of compost in agriculture. Salmonellae come from food wastes, especially from meats, poultry, milk and its derivatives. As seen in Figure 7, the average number of Salmonella was 5.6 MPN/4 g of dry solids at the beginning of the composting process and decreased to 2.8 MPN/4 g during the active phase (after only 20 days), and consequently covered the regulation limits for safe application of compost in agriculture.
Furthermore, as shown in Figure 7, Salmonella, total and fecal coliforms decreased significantly at the end of the composting period and reached to standard values. The computed correlation coefficients and the P values showed a significant correlation between the reduction of organic content and the microbial parameters (R2 = 0.92; P < 0.001). The decreasing trend of microbial biomass throughout the composting process (Figure 7) was in agreement with other works (47, 50).
Although there are several parameters used in monitoring compost maturity such as temperature, oxygen uptake rate, NH4/NO3 ratio and C/N ratio among others, germination index is one of the most reliable methods used in quantifying compost maturity. Germination index, which is a measure of phytotoxicity, has been considered as a reliable indirect quantification of compost maturity. It has been suggested that a germination index of 50% indicates the disappearance of phytotoxicity in composts (10).
At the present study, germination index was determined during the composting process. The values ranged between 11.43 to 96.17% from 0 to 80 days. Variations in the germination indices show that the rate of compost degradation differed at different times during the process. The value recorded in the composting mixture was initially low and equal to 11.43%, probably due to the continuing decomposition of organic matters that lead to the generation of phytotoxic compounds, such as volatile fatty acids, varied greatly between the days 20 - 50, but increased steadily from day 60. The rises and falls recorded in values between days 20 - 50 may be due to the different rates of decomposition occurring at the different positions of the compost masses and the persistent steady increases in values toward the end indicated the uniform decomposition at various points of the masses. In addition, the low percentage of germination index can be related to high EC of the compost mixture (5.6 ms/cm). Similar finding was reported by Ofosu-Budu et al. (51) on composting of municipal solid wastes. Nevertheless, the produced compost after 80 days has minimal phytotoxicity and can be safely used in soil with high degree of stability and maturity. In addition, Oviedo-Ocana et al. achieved similar results about stability and maturity of biowaste composts derived by small municipalities (52). Bernal et al. proposed that compost can be considered immature at GI values less than 80%, mature between GI 80% and 90% and highly mature at GI more than 90%. Nevertheless, it is noted that these threshold values highly depend on the type of seed used (53). Findings of Qian et al. showed that the GI values increased with the progress of composting and reached more than 100% after 90 days, signaling no phytotoxicity problem in the final compost (44).
4.4. Heavy Metals Content of Final Product
It has been known that metals cause a marked delay in germination, and that they can inhibit plant growth severely (54). Normally, some organic contaminants such as ammonia and phenol disappear during the composting process, but most of heavy metals tend to remain in the end-product; this constitutes a very important problem from an environmental and agricultural standpoint. Consequently, it is necessary to evaluate the concentration and phytotoxic effects of heavy metals in compost.
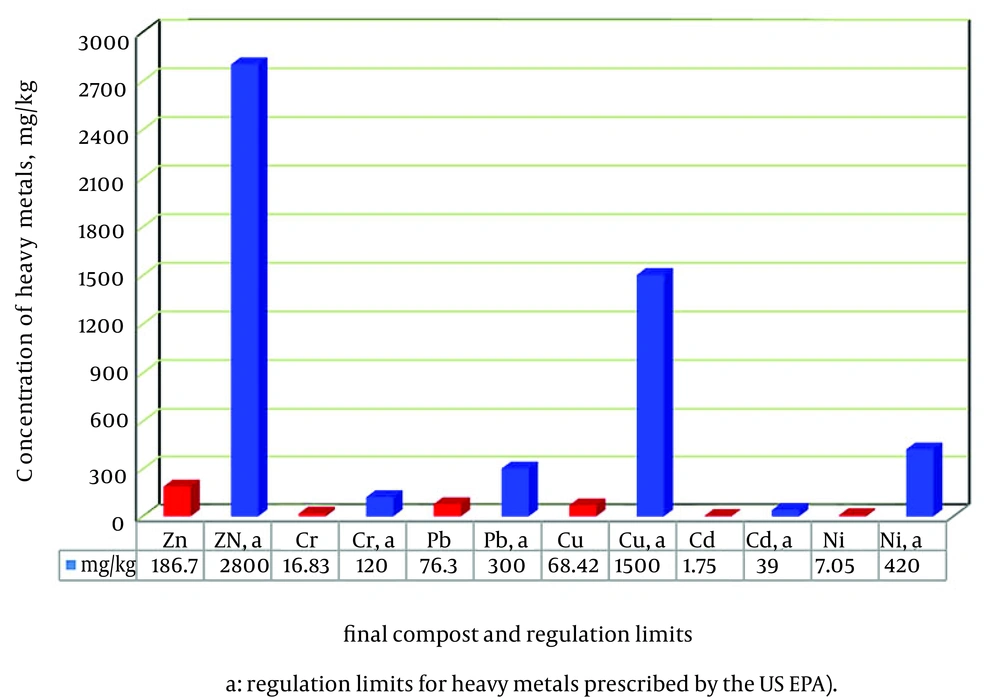
The concentrations of the selected heavy metals (Zn, Cu, Cr, Ni, Cd and Pb) in the mixture of compost are presented in Figure 8. As shown in Figure 8, the heavy metal contents in the refined materials were lower than the regulation limits prescribed by the US EPA for exceptional quality compost. The lead, copper and zinc concentrations were relatively higher than other heavy metals. The metal levels in the excavated product were comparable to mean metal concentrations in municipal solid waste compost reported in the literature (30, 55). As regulations are based on risk assessment, the potential for metals being a hazard in the use of this product may be limited (56). Of all potential quality standards for composts and biocomposts, heavy metals have been the focus of most considerations. Establishing and enforcing heavy metal standards is an effective approach to shield appropriate compost use (55).
5. Discussion
At the present study, maturity and stability of composted municipal solid wastes at a biocompost plant was evaluated. The C/N ratio decreased during the composting process (reached to 15.6), due to the loss of carbon and the increase in nitrogen content per unit material and also the NH4+/NO3 ratio decreased with increase in the time of composting and reached to 1.08. Furthermore, microbial parameters including Salmonella and total and fecal coliforms decreased significantly at the end of the composting period and reached to standard values. The computed correlation coefficients and the P values showed a significant correlation between the reduction of organic content and microbial parameters (R2 = 0.92, P < 0.001). GI increased during the composting process and the compost samples at the end of the composting phase had GI values greater than 90. In fact, the produced compost after 80 days had minimal phytotoxicity. In addition, all the heavy metals were well below the US EPA regulation ceilings. Lead, copper and zinc were at relatively higher levels than other metals when compared to regulation ceilings. According to the findings of this work and with respect to various maturity parameters, the produced compost in this study was mature and ready for use as an agricultural substrate or soil conditioner after 80 days of composting.