1. Background
Ultrasonic irradiation has been used for a variety of purposes (1). Inactivation of microorganisms is an essential step in water treatment as the final safeguard against water-borne microbial disease (2). There are the most widely physical and chemical technologies used for water disinfection (3). Chemical germicides are usually effective and relatively cheap, but can lead to the formation of hazardous organic by-products (especially in chlorination) (4). These methods are not environmentally friendly. Chemical methods are also limited by severe mass transfer limitations resulting in decreased disinfection rates (5). The potency of certain physical techniques, such as ultraviolet irradiation is limited in highly light scattering or absorbing solutions (6). On the other hand, some species of bacteria produce colonies and spores that agglomerate in spherical clusters. Application of biocide can destroy microorganisms on the surface of such clusters, but often leaves most of the inner bacteria intact. Flocs of fine particles can entrap bacteria and protect them against disinfection (7). Therefore, it is necessary to develop an advanced and eco- friendly method for inactivation of microorganisms in aquatic environments. Sonication is an eco-friendly alternative for water purification. The sound waves generated by this treatment can be utilized to eliminate microorganisms and organic pollutants in water.
Since human ear cannot detect the ultrasound waves because of their high frequency, they sound silent. Ultrasound Technology can be applied in gas, liquid and solid phase. The use of this process in liquid phase is called cavitations (8). In ultrasound waves, the vibration of the molecules in the environment, where the wave is being spread, transmits the energy (9). These waves produce strong cavitations in aqueous solution causing shock wave and reactive free radicals (OH, •HO2,•O) by the violate collapse of the cavitations bubble (3). These effects contribute to the physical inactivation of microbial structures and also the degradation of toxic elements (10, 11). Ultrasonic irradiation has been used for a variety of purposes (1). Ultrasound is able to inactivate bacteria and de-agglomerate bacterial clusters through a number of physical, mechanical and chemical effects (10). The lethal and biological effects of ultrasound were first reported in 1930s (12). In the 1960s researches concentrated on understanding the ultrasound mechanism in microbial inactivation (13). By 1975, it was shown that brief exposure to ultrasound caused thinning of cell walls, and attributed to the release of the cytoplasm membrane from the cell wall (14).
Ultrasonic process efficiency for elimination or inactivation of bacteria ,fungi, viruses and nematodes has been conducted in a number of studies which include E. coli (11, 12, 14-17), Pseudomonas aeroginosa and Staphylococcus (18), fungi (19, 20) and viruses (21). When ultrasound irradiation is coupled with ultraviolet light, it is able to achieve 97% - 100% removal of biological growth. Ultrasound at lower frequencies (20 - 100 kHz), classified as “power ultrasound”, and originally committed to water treatment, was applied to waste activated sludge disinfection for improving anaerobic stabilization. Low frequency has an important effect on the bacterial mortality (22, 23).
2. Objectives
This paper addresses the disinfection of drinking water using low frequency sonication in order to determine the fundamental effects of changes in cycle (power) and sonication time on E. coli and E. faecalis elimination. This study was innovative regarding the comparison of ultrasound efficiency on destruction of gram positive and gram negative water indicator bacteria, application of response surface methodology as a statistical method, and application of McFarland method with dilution and plate count.
3. Materials and Methods
3.1. Microorganisms and Inoculums Preparation
Experiments were conducted on laboratory scale using E. coli (ATCC 25922) purchased from Pasteur Institute of Iran and E. faecalis (ATCC 11700) bought from Department of Microbiology, the Iranian Research Organization for Science and Technology. For reviving Freeze-dried cultures of the selected bacteria, after striking the vials, 0.5 mL of azide dextrose broth was added to the freeze -dried cells with a sterile Pasteur pipette, and mixed properly. Then the total mixture was transferred to a vessel containing 500 mL of azide dextrose broth and incubated at 37 ˚C for 18 - 24 hours. Following incubation, 0.1 - 1 mL of incubated mixture was serially diluted with buffered peptone water (BPW) to obtain different dilutions and cell concentrations. These sets of assorted dilutions were used to identify the concentration of viable micro-organism in a fixed amount of a liquid by McFarland turbidity standards method (24). This method estimates the approximate amount of bacteria in suspension.
3.2. Sonication
Sonoplus ultrasonic homogenizer (CM2070) was applied with operating fixed frequency of 20 kHz and variable electric power output up to 70 w. The ultrasound treatment experiments were carried out separately under the same conditions for both selected bacteria.
3.3. Experimental Setup
Fifty mL drinking water samples were sonicated at 20 kHz and 70w (dissipated power “P diss ”) at different time lengths, cycles and selected bacterial concentrations (Table 1).
| Level of Variables | Cycle, pulse/s | Concentration, CFU/mL | Time, min |
|---|---|---|---|
| 1 | 2 | 3 | 5 |
| 2 | 6 | 6 | 7 |
| 3 | 10 | 9 | 9 |
Level of Variables
3.4. Statistical Analysis
To optimize runs and data analysis, Box-Benken statistical design, based on response surface methodology (RSM), was applied to investigate effects of the selected variables and minimize the number of experimental runs (25, 26). RSM is a developing and optimizing process in which the response of interest is influenced by several factors (27). Therefore, the effect of three explanatory factors (sonoplus ultrasonic homogenizer cycle, time and log of bacteria) on reduction of the selected bacteria was carried out using different types of cell suspension into seventeen experimental runs. An analysis of variance (ANOVA) was performed by Design-Experts 8 - Trial version. Also, the effect of ultrasound and regression coefficients of individual linear, quadratic and interaction term were measured for the bacteria.
3.5. Preparation of Bacteria for Test
For thawing E. coli (ATCC 25922), aseptically, 0.5 mL of TSB was added to the freeze-dried material, by pasture pipette, and mixed well. For E. faecalis (ATCC 11700), 0.5 mL of azide dextrose broth was used. The suspensions were transferred to an EMB agar slant for E-coli, and to a Pfizer selective Enterococcus Agar for E. faecalis. Finally, these cultures were incubated at 35 °C - 37 °C for 18 - 24 hours.
4. Results
4.1. Ultra Sound-Based Reduction of Escherichia coli (ATCC 25922) and Entrococcus Fecalis (ATCC 11700) Population in Suspension
The current investigation studied the effect of three main variables (time, cycle, and microbial concentration) to optimize ultrasound performance on inactivation of two indicator bacteria, E. coli and E. faecalis. The rejection values (response) at various experiments and conditions are shown in Table 2.
| Number | E. coli, SD | E. faecalis, SD | Run | Factor A Contact Time, min | Factor B Cycle, pulse/s | Factor C Log, CFU/mL | E. coli inactivation, % | E. faecalis inactivation, % |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 12 | 1 | 9.00 | 6.00 | 9.00 | 91 | 92 |
| 2 | 16 | 3 | 2 | 7.00 | 6.00 | 6.00 | 49 | 64.2 |
| 3 | 10 | 10 | 3 | 7.00 | 10.00 | 3.00 | 67 | 60 |
| 4 | 9 | 2 | 4 | 7.00 | 2.00 | 3.00 | 41 | 43 |
| 5 | 17 | 9 | 5 | 7.00 | 6.00 | 6.00 | 47 | 63.1 |
| 6 | 14 | 16 | 6 | 7.00 | 6.00 | 6.00 | 50 | 65 |
| 7 | 3 | 8 | 7 | 5.00 | 10.00 | 6.00 | 32 | 42 |
| 8 | 1 | 17 | 8 | 5.00 | 2.00 | 6.00 | 10 | 32 |
| 9 | 7 | 11 | 9 | 5.00 | 6.00 | 9.00 | 27 | 34 |
| 10 | 12 | 15 | 10 | 7.00 | 10.00 | 9.00 | 71 | 75 |
| 11 | 5 | 14 | 11 | 5.00 | 6.00 | 3.00 | 16 | 30 |
| 12 | 11 | 7 | 12 | 7.00 | 2.00 | 9.00 | 50 | 44 |
| 13 | 13 | 6 | 13 | 7.00 | 6.00 | 6.00 | 52 | 58 |
| 14 | 15 | 1 | 14 | 7.00 | 6.00 | 6.00 | 51 | 62 |
| 1516 | 6 | 5 | 15 | 9.00 | 6.00 | 3.00 | 93 | 87 |
| 17 | 2 | 4 | 16 | 9.00 | 2.00 | 6.00 | 81 | 82 |
| 18 | 4 | 13 | 17 | 9.00 | 10.00 | 6.00 | 99.99 | 97.5 |
Response Value at Various Variables for Escherichia coli and Enterococcus faecalis
As it can be seen in Table 2, the maximum reduction for E. coli was 99.99% (4 log) with P < 0.0001 and for E. faecalis was 97.5% with P = 0.0002 at 10 pulse/s in 9 minutes and 6 log CFU/mL bacterial suspension. Application of US technology for disinfection process was described in some articles (28, 29). The lethal effect of ultrasonication was also reported for reduction of Yersinia enterocolitica, Bacillus subtilis spores, Listeria monocytogenes, Salmonella spp, Aeromonas hydrophila, Legionella pneumophila, Acanthamoeba castellanii, Saccharomyces cerevisiae, Pseudomonas aeroginosa and Staphylococcus aurous (4, 18, 25, 30, 31).
4.2. Response Surface Modeling for Escherichia coli and Enterococcus faecalis
Response surface Methodology (RSM) is an important branch of experimental design and a critical technology in developing new processes and optimizing their performance. The objective of quality improvement, including reduction of variability, improved process and product performance, can be often accomplished directly using RSM. Regression analyses of the experimental data with ANOVA, and results of the quadratic models for E. coli and E. faecalis, were summarized in Table 3 and 4.
| Source | Sum of Squares | df | Mean Square | F value | P value, Probability> F value | Significance Level |
|---|---|---|---|---|---|---|
| Model | 11050.40951 | 11 | 1004.582682 | 298.9829412 | < 0.0001 | Significant |
| Time (A) | 9799.300013 | 1 | 9799.300013 | 2916.458337 | < 0.0001 | |
| Cycle (B) | 967.7800125 | 1 | 967.7800125 | 288.0297656 | < 0.0001 | |
| Log, CFU/mL (C) | 135.7050066 | 2 | 67.85250329 | 20.19419741 | 0.0040 | |
| AB | 2.265025 | 1 | 2.265025 | 0.674114583 | 0.449 | |
| AC | 42.7550125 | 2 | 21.37750625 | 6.362353051 | 0.0423 | |
| BC | 10.7650125 | 2 | 5.38250625 | 1.601936384 | 0.2900 | |
| A2 | 31.23711184 | 1 | 31.23711184 | 9.296759477 | 0.0285 | |
| B2 | 43.75816447 | 1 | 43.75816447 | 13.02326324 | 0.0154 | |
| Residual | 16.8 | 5 | 3.36 | |||
| Lack of fit | 2 | 1 | 2 | 0.540540541 | 0.5030 | not significant |
| Pure error | 14.8 | 4 | 3.7 | |||
| Cor total | 11067.20951 | 16 |
Analysis of Variance Results of Response Surface Quadratic Model for Escherichia coli Inactivation
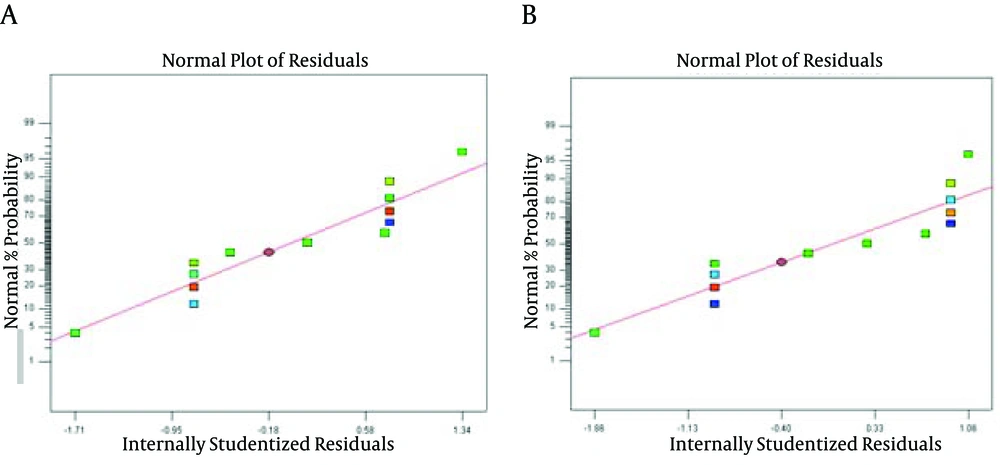
A, B and C are coded variables for sonication time, sono-reactor cycle and initial cell concentration, respectively. The normal probability plots of the studentized residuals for the selected bacteria are shown in Figure 1. According to Figure 1, the data for both bacteria were normally distributed. In these graphs, residual value indicates the difference between the obtained response and the fitted value under the theorized model. Therefore, the model was adequate for prediction within the range of experimental data.
| Source | Sum of Squares | df | Mean Square | F value | P value Probability> F value | Significance Level |
|---|---|---|---|---|---|---|
| Model | 7114.521824 | 11 | 646.7747112 | 89.53881984 | < 0.0001 | Significant |
| Time (A) | 6077.53125 | 1 | 6077.53125 | 841.3671194 | < 0.0001 | |
| Cycle (B) | 675.28125 | 1 | 675.28125 | 93.48523548 | 0.0002 | |
| Log, CFU/mL (C) | 174.8326053 | 2 | 87.41630263 | 12.10182222 | 0.0121 | |
| AB | 7.5625 | 1 | 7.5625 | 1.046944652 | 0.3531 | |
| AC | 11.53125 | 2 | 5.765625 | 0.798187142 | 0.5002 | |
| BC | 112.28125 | 2 | 56.140625 | 7.772049866 | 0.0292 | |
| A2 | 40.00760526 | 1 | 40.00760526 | 5.538611355 | 0.0653 | |
| B2 | 19.78128947 | 1 | 19.78128947 | 2.738501187 | 0.1589 | |
| Residual | 36.117 | 5 | 7.2234 | |||
| Lack of fit | 6.125 | 1 | 6.125 | 0.816884503 | 0.4172 | not significant |
| Pure error | 29.992 | 4 | 7.498 | |||
| Cor total | 7150.638824 | 16 |
Analysis of Variance Results of Response Surface Quadratic Model for Enterococcus faecalis inactivation
4.3. Predicted Second Order Polynomial Equations
The predicted second order polynomial equations, after neglecting statistically non-significant values for the selected bacteria, are given in Table 5.
| Response | Equations | P value | R2 |
|---|---|---|---|
| Escherichia coli (Y1) | Y1 (%) =50.38+8.57*A+1.05*B+16.00*C[1]+0.071*C[2]-0.094*AB-1.62*AC+0.084* AC - 0.31 * BC+0.13* BC+0.68* A2+0.20* B2 | < 0.0001 | 99.85 |
| Enterococcus faecalis (Y2) | Y2 (%)= 59.27+27.96* A+10.13 * B+3.13* C[1]-1.60 * C[2]+1.37 * AB +0.25 * AC[1]+0.79* AC[2]+3.50* BC[1]+1.88* BC[2]+3.08*A2-2.17 * B2 | < 0.0001 | 99.49 |
Predicted Second Order Polynomial Equations for the Selected Bacteria a
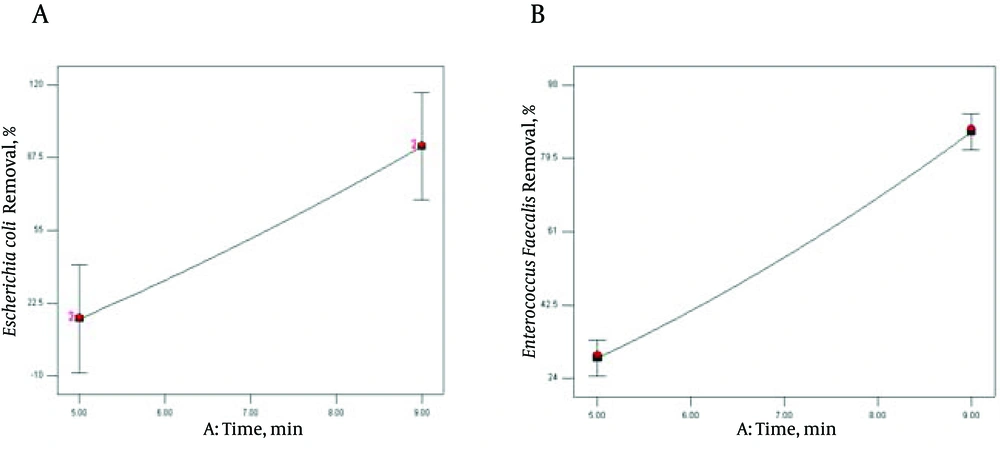
Sonication time (A) exhibited the significant interaction with E. coli initial concentration (P < 0.0423) to affect reduction of E. coli population by ultrasound radiation, while no statistically significant changes were found among the interactions between cycle with E. coli concentration (P < 0.290) and with time (P < 0.449). The second order effect of time (A2) and E. coli concentration (B2), were also significant (P < 0.0285 and P < 0.0154), respectively. However, the effect of A2 was significantly (P = 0.0285 & F = 9.30) lower than that of B2 (P = 0.0154 and F = 13.02) in E. coli inactivation, based on statistical indices and visual observations (Table 3). According to the obtained results, sonication cycle and E. faecalis concentration had significant interaction in the rate of E. faecalis inactivation (0.0292) (Table 4). It is clearly observed that E. coli and E. faecalis reduction increased with increasing the contact time (A) and cycle (B). In contrast, selected bacterial initial cell concentrations had negative effect on population reduction. According to Figure 2, sonication time was significant (P < 0.0001) for E. coli and E. faecalis removal efficiency.
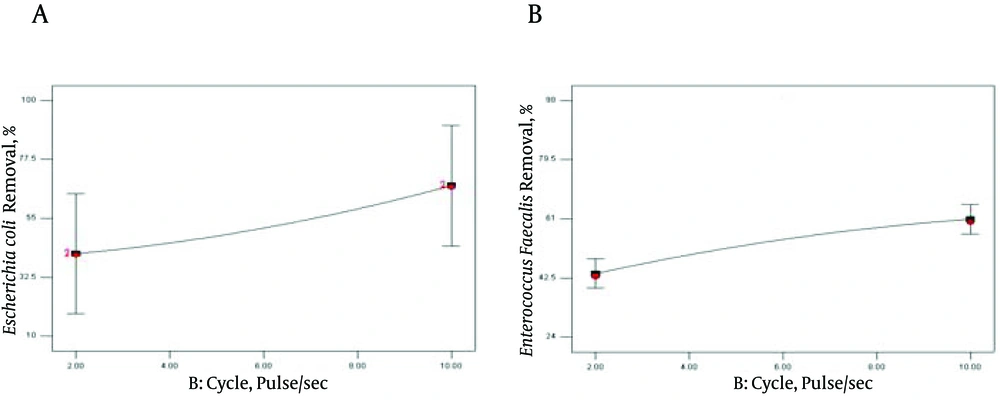
In addition, the effect of sonicating on E. coli and E. faecalis suspension at 3 level cycles (pulse/s) is demonstrated in Figure 3.
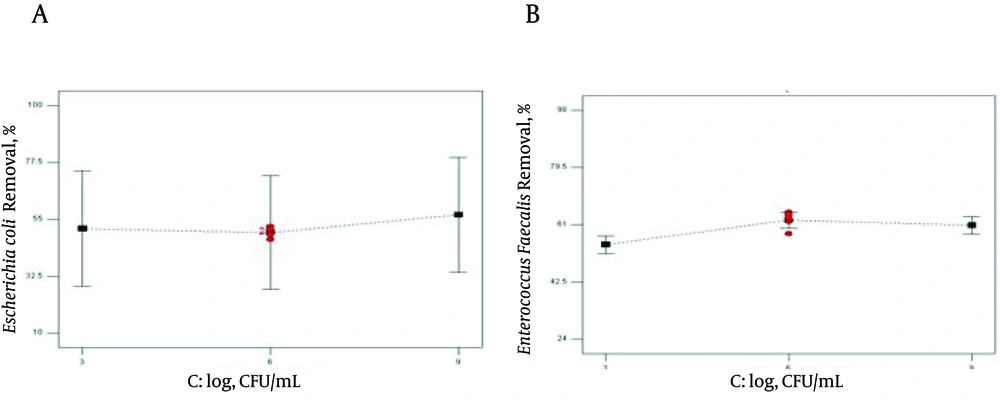
Three different initial cell numbers (3, 6, 9 log CFU/mL) were used in our experiments. The effect of bacterial log on reduction rate of E. coli and E. faecalis was demonstrated in Figure 4.
5. Discussion
5.1. Ultra Sound-Based Reduction of Escherichia coli (ATCC 25922) and Enterococcus faecalis (ATCC 11700) Population in Suspension
The maximum degradation of bacteria in polluted waters using ULS for pretreatment has been reported in the study by Ince et al. (32). The US process for bacterial removal is based on acoustic cavitations. These mechanisms include chemical attack by hydroxyl radicals produced by the USA, cell death because of high pressure and temperature caused by bubble collapse, and shared forces that destroyed bacterial cell membrane (33). The free radical is particularly important in biodegradation and microbial inactivation (30, 34, 35). Exposing high mechanical pressure waves to liquids creates an acoustical stream and subsequent acoustic cavitations that cause formation, growth and implosive collapse of micro and nano-bubbles in a liquid. These bubbles have a large surface area which increases the diffusion of gas and generates intense localized heating (approximately 5000 °C) and high pressure (1000 ATM) (17, 22, 36, 37).
Ultrasonic cavitation affects inner membrane (the cytoplasmic membrane) of bacteria and lipoprotein bi-layer is disrupted, torn and shredded. Ultrasound does not create an immediate cellular membrane rupture. In contrast, oxidizing biocide mechanism causes immediate cellular ATP release. ATP measurement indicated that due to perforation of cell wall, the ratio of extra/intra cellular ATP increases under sonolysis (38). Thickness of cell membrane has mainly affected microbial removal efficiency (39) that is why ultrasound irradiation affects E. coli (99.99%) more than E. faecalis (97.5%). E. faecalis is a gram positive bacteria and its cell wall is thicker than that of E. coli. A few solutions have shown that gram-negative bacteria such as E. coli are more susceptible to this treatment than gram-positive bacteria. Even, E. coli indicated higher sensitivity than the total coliforms (40). The results of ANOVA in Tables 3 and 4, high coefficient of correlation (R2 = 99.85 for E. coli and R2 = 99.49 for E. faecalis) along with non-significant lack of fit (P > 0.05 indicated that the model was reproducible. The coefficient of variation (CV%), 3.36 for E. coli and 4.43 for E. faecalis, which were less than 5%, also supported and confirmed the importance and validity of the model. It means that this model is in support of our hypothesis i.e. the relationship between sonication method and bacterial inactivation. The significance and adequacy of the model are also demonstrated by F value (Fisher Variation ratio), probability value (P value) and adequate precision. The ANOVA results also proved the validity of quadratic model with probability > F value of less than 0.0001 for E. coli and 0.0001 for E. faecalis.
5.2. Effect of Treatment Time
According to ANOVA tables (Table 3 and 4) and Figure 2, the P value of linear coefficient of time (A) for sonication time were significant (P < 0.0001) in both of the equations for E. coli and E. faecalis. During the ultrasound treatment, 4 log reductions of E. coli and E. faecalis cells were achieved at 9 minutes. Lee et al. achieved up to 4 log reductions in 4 min sonication (30). In Munoz et al. report, 5 minutes contact time is required to achieve a one log reduction of E. coli at 55 °C in orange juice by thermosonication and high intensity light pulses (41). Similar observations in bacterial inactivation have been reported to increase residence time from 80 to 120 and 160 seconds (7). As the time of sonolysis increases, the temperature in the volume of solution rises and accelerates the diffusion processes of ions in the cell membranes (35). As a results, during US irradiation, the decomposition of pollutants in solution occurs under the effect of the oxidizing process by produced OH* radicals due to thermal destruction, and US dynamic mixing and sharing pressure (23, 35). Dehgani et al. found that the fungi population decreased with Increasing sonication time (20). The influence of ULS dose on E. coli inactivation was studied at two different sonolysis time lengths (15 and 30 minutes). The results showed a synergistic effect of US on E. coli reduction at 15 minutes and higher US dose (15).
5.3. Effect of Sonolysis Intensity
The effect of sonicating on E. coli and E. faecalis suspension at 3 level cycles (pulse/s) is demonstrated in Figure 3. As expected, there was approximately linear increase in the inactivation of the selected bacteria, as the sonication cycle increased from 2 to10 pulse/s (Figure 3). However, comparing the slope of curves in Figure 2 and 3 demonstrated that the effect of time was significantly more than that of cycle (pulse/sec) on bacterial reduction. During pulse intervals, the active surface of bacteria had more time to adsorb ULS cavitations bubbles leading to more inactivation (42). According to the data in Marques et al. studies, in 20 kHz frequency and intensity of 10 W/cm2, they observed a large increase in the phosphatase and ATPase activity (43). Lanchun et al. (2003) results confirmed the effect of ULS irradiation on enzymatic activity of microorganisms (44). However, high intensity of ULS may be conducted to obtain 100% killing rate of microorganisms (3). Significant effect of ULS high intensity on total coliform and heterotrophic bacteria in waste water sediments has been noted in some researches (45). On the other hand, some investigations have shown the favorable effect of low intensity on plants (46). Some researchers established that low ULS power did not affect the primary physiological characteristics of microorganisms (44). The ULS intensity directly depends on ULS wave amplitude (47). The results of using ULS with different amplitude indicated that increasing the ultrasound amplitude mainly affects microbial reduction (5, 44, 48, 49).
5.4. Effect of Specific Energy on Removal Efficiency
The ultrasonic specific energy (Es, kJ/L) was calculated by Equation 1:

Where, P diss is dissipated ultrasound power in the samples, t is ultrasound irradiation time (s), V is the volume of sample (L) (2). In the present study, P diss = 70 W and V = 50 mL and 5 - 9 time range, therefore specific energy (ES) was obtained in the range of 70 - 126 kJ/L. Specific energy (ES), as a reference parameter, specified the relationship between irradiation time, power and treated volume (16). It can be clearly noted that the rate of bacterial removal increases with increasing ultrasound Specific Energy (50).
5.5. Effect of Initial Cell Number on Escherichia coli and Enterococcus faecalis Removal Efficiency
As observed in Table 3 and 4 together with Figure 4, the effect of the selected bacterial concentration on removal efficiency was less than those of the other variables (P = 0.004 for E. coli and P = 0.0121 for E. faecalis). Maximum removal efficiency for the selected bacteria was obtained in 6 log CFU/mL. Although P value < 0.05 corroborates the statistical significance of this variable, Figure 4 demonstrated that initial concentration of E. coli has no efficient effect on bacterial reduction (51). In contrast, Tsukamoto came to a conclusion that initial log numbers of bacteria strongly influence the reduction rate, and the lowest concentration was the most effective factor on removal efficiency (19). According to Bigelow et al., sonication was able to completely remove the E. coli biofilms at highest exposure level (52). The results showed that a higher initial bacterial concentration needed a larger sonication time to obtain the best bacterial reduction (16). When the initial E. coli concentration increases, the •OH radical concentration acts as the limiting factor of the disinfection processes (53). The current study demonstrated the sonolytic inactivation of E. coli, (ATCC 25922) and E. faecalis (ATCC 11700). The findings showed that, high treatment time is capable of eliminating the E. coli and E. faecalis in the solution, almost completely. However the effect of ultrasound irradiation on E. coli because of high sensitivity was more than that of E. faecalis. Besides treatment time, other variables that affect bacterial disruption efficiency are ultrasound cycle and initial bacterial log. Finally, it was demonstrated that bacterial inactivation increased by sonication.