1. Background
Polystyrene is an aromatic organic solvent that is widely used in many industries, including plastic, synthetic rubber, packaging, toys, and furniture production (2, 3). Exposure to the excessive levels of styrene may lead to irritation of mucous membranes and eyes, dermatitis, and acute and chronic effects on the peripheral and central nervous systems (CNS) (4, 5). A large number of studies have reported the harmful effects of styrene on the CNS, such as depression, color discrimination, and nerve conduction velocity (6, 7).
Long-term uncontrolled exposure to loud noise increases the risk of developing hearing loss. In addition, physiological problems, impairment of the performance, mood disorder, fatigue, discomfort, and in the following the reduction of muscle movement are non-auditory effects of noise exposure (8-10). Animal studies have shown that exposure to noise with moderate-intensity levels exerts negative effects on learning and memory in the water maze test, whereas noise with higher intensity causes more significant and destructive effects (11).
It has been shown that acute or chronic exposure to high-intensity noise damage learning and memory of rats (1). In addition, noise exposure during pregnancy leads to mental retardation, impairment of the learning, and hippocampal activity in newborn rats (12). Naqvi et al. investigated the acute effect of noise on rat behaviors. They found that the impairment of locomotor activity and increased anxiety is relatively a consequence of noise exposure (9).
Some animal studies have reported a synergistic interplay between the effects of noise and solvent exposure (13, 14). Many of these studies have focused on the auditory system (15, 16) but results about behavioral and neurochemical effects are rare and inconsistent. Subchronic combined exposure to noise and toluene (16 weeks, 104 h/week; 80 dB-A, 8 kHz; 40 ppm) showed no additional behavioral and neurochemical adverse effects (17). Morton et al. reported that a synergistic interaction between noise and toluene may alter brain functions (17). Background noise (90 dB) significantly suppressed brain activity and this suppression was exacerbated with solvent exposure (18).
Since workers in many industries are exposed to noise and high concentrations of organic solvents such as styrene (17) and little is known about the co-exposure effects of noise and styrene on behaviors and cognition; therefore, in the current study, we investigated the effect of noise and styrene on memory and behaviors of rats using Y-maze and open field test. The Y-maze test is used to investigate rodent spontaneous alternation behavior and also assess the normal hippocampus-dependent navigation behavior (19, 20). The open field test is designed to measure behavioral responses such as locomotor activity and exploratory behavior (21).
2. Methods
2.1. Animals
For conducting this experiment, male Wistar rats weighing 250 - 300g (Pasteur Institute, Tehran, Iran) were used. The animals were placed within the polypropylene cages (40 cm × 25 cm × 15 cm), with 12/12-hour light/dark cycles. The animals were freely accessed to water and food in their home cage. All experiments were performed in accordance with the guide for the care and use of laboratory animals (National Institutes of Health Publication No. 80 - 23, revised 1996) and were approved by the Research and Ethics Committee of Tarbiat Modares University.
2.2. Experimental Protocol
In this study, we investigated the effect of noise (80 - 120 dB) (15, 22) and styrene (50 to 1000 ppm) (23, 24) on the working memory and locomotor activity. In another part of the study, we examined the effect of simultaneous exposure to noise (100 dB) and styrene (750 ppm concentration) on working memory and locomotor activity in male rats.
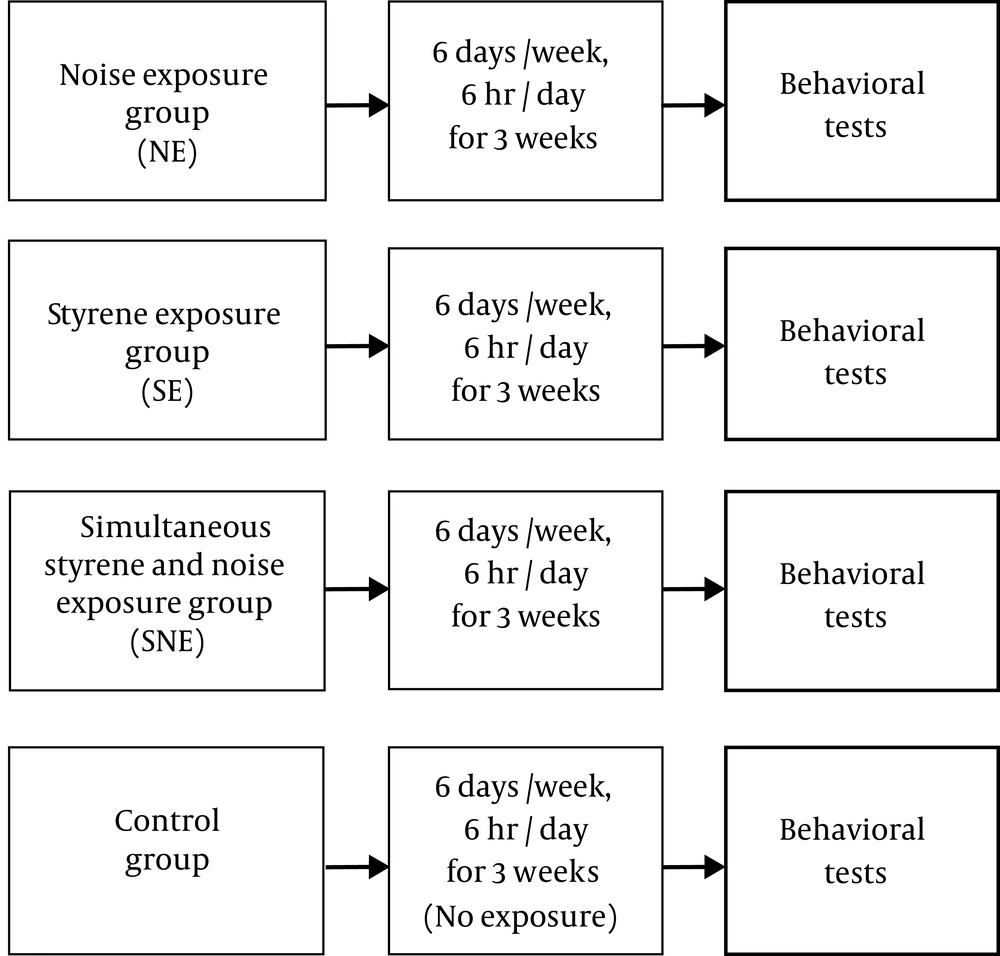
Animals were randomly divided into 4 groups: the noise exposure (NE), the styrene exposure (SE), the simultaneous styrene and noise exposure group (SNE), and the control group. Each experimental group consisted of 6 - 8 animals. NE animals were exposed to bandpass white noise entered at 8 kHz (100 dB), the SE group exposed to styrene (750 ppm), and SNE group simultaneously subacute exposed to styrene and noise (750 ppm styrene and 100 dB noise). The period of the exposure was 3 weeks (6 days/week, 6 hours/day). The audio frequency was within the most sensitive frequency areas in the rat (25). At the end of the exposure period, working memory and locomotor activity were evaluated by Y-maze and open field test, respectively (8) (Figure 1).
2.3. Exposure to Noise
The noise was generated on a computer by Timo Esser’s Audio software (version 1.2), recorded and played by the Cool Edit Pro software (Syntrillium Software Corporation, version 2.1), amplified by an audio amplifier (Pejvak Ava Corporation, Model AP12), and delivered by loudspeakers (JBL GT6-6) that were located 12 cm above the wire cages. By using a sound level meter (Casella CEL 480), the level of noise was measured. The noise levels varied less than ± 1 dB (100 ± 1 dB) between the measuring points and the frequency distribution of the noise matched with high-pass white noise spectrum.
2.4. Exposure to Styrene
Rats were exposed to 750 ppm styrene (above 99% purity) (Daejung Chemicals and Metals Co., LTD), using a dynamic exposure chamber (16). The purity of saturated styrene vapor was diluted and the styrene concentration in the chamber was continuously monitored every 20 minutes with Miran 1A Pho Check (Wilks Scientist Corp., USA). Also, the styrene concentration was incessantly monitored in the chambers. In the inhalation chamber by using a gas chromatograph, the temperature was 20 to 24°C, humidity 55%, and styrene concentration 750 ppm.
2.5. Open Field Test
In the present study, the open field test was used to evaluate locomotor activity and anxiety. A black rectangular box of wood (60 cm × 60 cm × 60 cm) exists in the open field apparatus (9). The animals were put in the center of the box and allowed to independently move into the area for 15 minutes. The behavior of the rats was recorded using NeuroVision software. Movements of each animal were recorded for 15 minutes by a video camera. Subsequently, the apparatus was thoroughly cleaned with 70% ethanol for each animal. By using the software the open field box was divided into 25 squares. The time spent in the inner zone of the arena was measured as an indicator of lack of anxiety, and the anxious rats usually represent freezing action and stay near to the sides of the arena.
2.6. Y-Maze Test
Y-maze was used to evaluate spontaneous alternation behavior. This behavior is a criterion for measuring hippocampus-dependent working memory (19). Y-maze tests were conducted using Y-shaped maze consisted of three black, compressed plastic arms (length 50, width 16, height 32 cm) at a 120° angle from each other.
After the introduction to the center of the maze, the animal was allowed to freely explore the three arms for 10 minutes. Over the course of multiple arm entries, the subject should display an attitude to enter a less lately visited arm. The number of arm entries and the number of triads were recorded in order to compute the percentage of spontaneous alternation. An entry occurs when all four limbs were within the arm. This test is used to quantify cognitive deficits in transgenic strains of rat and appraise novel chemical entities for their effects on cognition. Spontaneous alternation behavior percentage was computed according to the following formula:
[(The number of correct alternations/number of arm entries - 2)] × 100
2.7. Statistical Analysis
The data are expressed as mean ± SEM (standard error of mean). The data were processed by the software SPSS V. 21. Statistical analysis was executed using one-way ANOVA followed by post-hoc analysis (Newman-Keuls test). P values less than 0.05 were considered statistically significant.
3. Results
3.1. Locomotor Activity
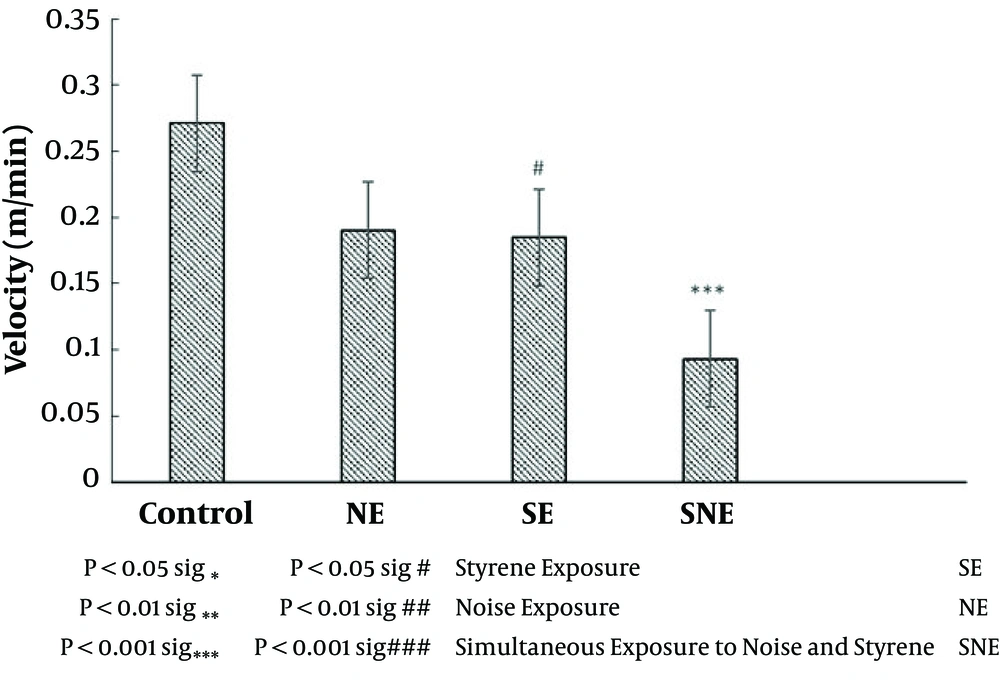
Figure 2 presents the velocity as an indicator of locomotor activity in the open field test. Statistical analyses showed that simultaneous exposure to noise and styrene (SNE group) significantly (P < 0.0001) decreased locomotor activity compared to the control group (Figure 3). In addition, styrene or noise exposure had no effect on locomotor activity compared to the control group. Locomotor activity was significantly decreased in the SNE group compared to the SE group (P < 0.05), while there was no significant difference between the SNE and NE groups.
3.2. Anxiety
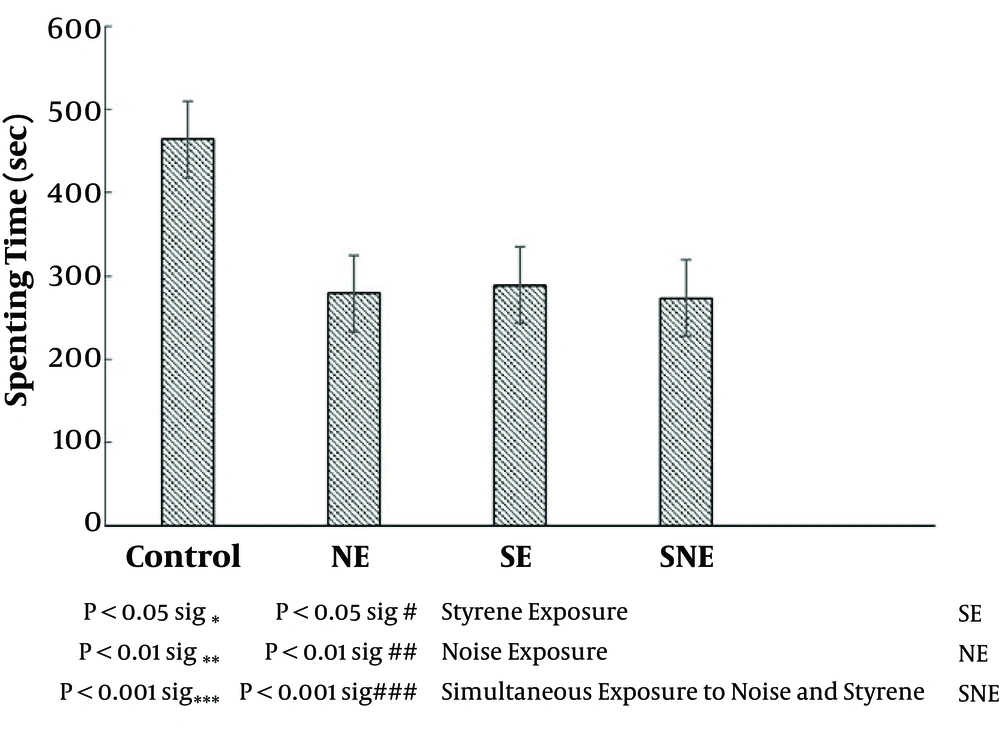
The time spent in the center zone was considered the lack of anxiety indicator. Our results showed that the exposure to noise, styrene, and simultaneous exposure to styrene and noise decreased time spent in the center zone, but the difference was not significant in comparison to the control group (Figure 3).
3.3. Working Memory
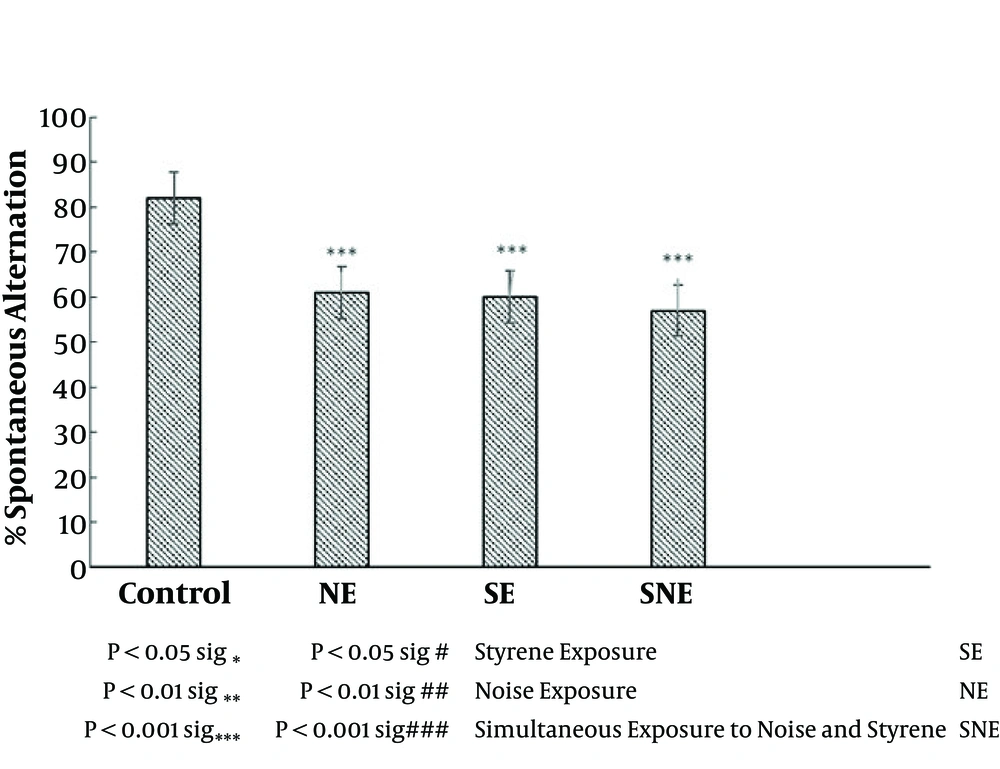
To evaluate the effect of noise and styrene on working memory we carried out these experiments. The spontaneous alternation was considered an index of working memory. Exposure to noise, styrene, and simultaneous exposure to styrene and noise significantly decreased working memory compared to the control group (P < 0.0001). There was no significant difference in terms of working memory in the SNE group compared to the SE and NE groups (Figure 4).
4. Discussion
4.1. Effect of White Noise on Locomotor Activity, Anxiety, and Working Memory
The results showed that simultaneous exposure to noise and styrene decreased locomotor activity, while styrene or noise exposure alone had no significant effect on locomotor activity. Haider et al. have shown that sub-chronic exposure to noise decreased the locomotor activity, whereas increased the concentration of dopamine in the brain and the rate of monoamine transmission (21).
Anxiety is a physiological and behavioral state, which can be induced by stress and characterized by fear, annoyance, and neurochemical changes in the brain (9). In the present study, exposure to noise, as a stressor, did not affect anxiety. Naqvi et al. showed that chronic noise exposure induces exhaustion, defeat, and anxiety (9). The distinction between the findings may be due to the different exposure protocols and the fact that anxiety levels were assessed by elevated plus maze test while we only used the open field test.
In addition, the results showed that sub-acute exposure to noise impaired the working memory of the rats. Recently, some evidence has shown that chronic noise exposure will contribute to cognitive impairment, which is presented with declined memory and learning (26, 27). Noise exposure impaired the learning and memory in mice in the Morris water maze model (10). Liu et al. found that noise exposure (100 dB) for 10 days could reduce learning, reduced the number of neurons, and caused neural abnormality (10). Chronic noise stress reduced dendritic count in the hippocampus and cortex in rats that eventually impaired working memory (12). In addition, exposing to noise (100 dBA/4 hours per day) produced oxidative stress in rats (11). Long-term noise-induced oxidative stress in rats increased acetylcholinesterase activity, reduced dendritic count in the hippocampus, and elevated plasma corticosterone level, which might cause the impairment of memory (11).
4.2. Effect of Styrene on Locomotor Activity, Anxiety, and Working Memory
The results revealed that sub-acute exposure to styrene had no effect on locomotor activity in rats. In agreement with our results, Husain et al. reported that exposure to styrene had no effect on dopamine content (28). Hougaard et al. observed that exposure to 1800 ppm of toluene by gestation (7 - 20 days, 6 hours/day) had no effect on the activity of pups (29). Some results are inconsistent with our results, for example, Chakrabarti et al. studied the effects of styrene by gavage on locomotor activity and concluded that exposure to styrene decreased locomotor activity and brain dopamine concentrations (30). Also, the study showed that inhalation of styrene for 7 days impaired motor coordination and delayed in the movement rat (31).
Another part of the study showed that exposure to styrene increased the anxiety behavior of rats. Husain et al. reported oral intubation of styrene in male rats increased serotonin levels (32). The role of serotonin (28, 33) and dopamine (21) in the pathophysiology of anxiety has been clearly proven. Phenylglyoxylic acid as one of styrene metabolite had no effects on neurobehavior after using a functional observational battery or radial arm maze and increased dopamine concentration in the cerebellum, hippocampus, pons, and whole brain (34).
In a study that performed on clinical and neurobehavioural effect of styrene on the worker, any significant differences in the relevant neurobehavioural variables between the styrene workers and the controls were not observed (35). In our study, the exposure to styrene impaired working memory. Because most organic solvents such as styrene are volatile liquids and also lipophilic, they easily bind to lipid-rich tissues such as the brain (31). In another study, Chery et al. reported that 23% of workers exposed to less than 50 ppm to 71% of those exposed to more than 100 ppm of styrene have deficits in the peripheral and central nervous systems (36).
4.3. The Interaction Between the Noise and Styrene Exposures
Simultaneous exposure to styrene and noise decreased locomotor activity compared to the control group. Several pieces of evidence have mentioned that noise can affect the brain neurochemistry, for example, some studies have reported that exposure to noise changed the brain level of serotonin and dopamine (21, 37, 38). On the other hand, the impairment of turnover ratios of serotonin and dopamine has also been reported following styrene exposure (30, 32). Such a decline in locomotor activity may be attributed to alterations of brain structure and neurochemistry such as changing serotonin and dopamine levels. Simultaneous exposure to noise and styrene did not cause anxiety-like behavior in rats; however, we expected that it might induce anxiety-like behavior.
In addition, the results indicated that simultaneous exposure to noise and styrene impaired working memory compared to the control group. Inconsistent with our results, combined exposure to noise and toluene (16 weeks, 104 hours/week; 80 dB-A, 8 kHz; 40 ppm) had no additional behavioral and neurochemical adverse effect (39). Evidence indicates that oxidative stress, which is caused by noise stress, may cause lipid peroxidation and cognitive decline (27). On the other hand, styrene in the body often metabolized to styrene 7, 8-oxide, which can cause direct oxidative stress of cells (40). Free radical-mediated operation through different pathways, inducing directly or indirectly to neurodegeneration and behavioral changes (1). Brain cells compared to other parts of the body cells are very vulnerable to lipid peroxidation because they have high saturated fatty acids as well as high molecular oxygen (1). Consequently, owing to the similarity of the mechanism of both factors on the brain, it may be expected that simultaneous exposure had a worse effect.
4.4. Conclusions
Our study showed that simultaneous exposure to styrene and noise have destructive effects on cognition and behaviors. The exposure to styrene and noise should be taken into consideration in the workplace and it is necessary to avoid the exposure of workers to these factors. However, further clinical and molecular studies are needed to clarify the effects of styrene and noise on human and also the accurate mechanisms involved in its destructive effects.