1. Background
In recent years, the adverse health effects of allergens in outdoor air have received a great deal of scholarly attention (1-3). The presence of fungal spores in different environments is very important since it is related to various types of free airborne allergens (4). Exposure to outdoor air fungal spores is associated with allergic respiratory symptoms and decreased lung function (5). One of the most important sources of airborne particles is dust storms, which in recent years have caused many problems in many parts of Iran, especially in the west and southwest dust storms; therefore, they are very important in the air quality management, because their suspended particles can maintain long distances by retaining their nature. However, the time of suspension in the atmosphere varies by parameters such as particle size, regional conditions, wind speed, wind time, rainfall and altitude (6). Dust storms can contain a variety of bioaerosols. Fungi and their spores are among these bioaerosols (7, 8). Contact with bioaerosols is associated with a wide range of health problems, including acute toxic effects, allergies, and cancers (9). Most fungi can create spore-forming resistance to drought, heat, ultraviolet radiation and nutrient-poor conditions. Each gram of desert soil contains 106 fungi. The highest number of fungal spores measured in dust storms is 106 spores per cubic meter in tropical areas. Fungi are among organisms that pollute the air, and under certain conditions, have the potential for pathogenicity in humans or animals. A normal person during one-day breathing enters about 360 million fungal spores in their lungs. Spores that spread through the air can be long-lasting and contaminate different surfaces, and the spores deposited on surfaces are capable of re-emerging in the air (10, 11). Almost more than 2,000 fungal species are not infectious for humans, but they are the most opportunistic pathogens. These fungi easily endanger people with disabilities and immune deficiencies due to the ability of adaptation to environmental conditions (12). The complications that fungi have on human health depend on the structure of an individual’s immune system, type of contact, and microorganism nature (13). Saprophytic fungi are the most common fungi and important biological agents (bioaerosols) that pollute the environment and the air. Some fungi cause superficial diseases such as Otomycosis and Keratomycosis. Some of them, e.g. Aspergillus, Penicillium, and Alternaria are allergens and increase sensitivity to asthma, allergic rhinitis, and pneumonia in humans. Serology is the best way to investigate allergies and complications of airborne fungi as an antigen in the air. For this reason, the identification of common allergen fungi in a region under different environmental conditions would help health professionals involved in infectious diseases, skin specialists, etc., in preventing and treating diseases caused by human contact with fungi (14). Atmospheric and topographic conditions of the region and bioavailability of bioaerosols affect their survival in the environment (14, 15). Air fungi have been studied in most parts of the world (16, 17). Iran has been no exception and studies have been carried out in different cities such as Tehran, Isfahan, and Zahedan (18).
2. Objectives
This study aimed to determine the effect of dust storms on fungi spore’s diversity in hot and cold seasons in Ahvaz, Iran.
3. Methods
3.1. Description of Study Area
This study was a cross-sectional study aimed to assess the fungal diversity and the number of fungal agents and also to examine their significant differences on normal and dust event days in three regions of Ahvaz, Iran, from July to December 2017 have been selected. Station 1 (Amanyeh) as the high traffic area, station 2 (University campus) as the area, and station 3 (Golestan) as the residential area. Ahvaz, with a population of approximately 1 million residents and an area of 8,152 km2, is the capital of Khuzestan province. It is located between 48 degrees to 49°29’ east of Greenwich meridian and between 31º and 45’ minutes to the north of the equator. The study area is shown in Figure 1.
3.2. Fungal Examinations
A total of 93 samples were collected and then analyzed. The samples were collected between 18 to 20 o’clock with Andersen N6 samplers (Thermo Andersen, Inc., Atlanta, Ga.) using Potato Dextrose Agar (PDA, Merck, Germany) as the culture medium. The sample collection of bioaerosols from the three areas on normal days was performed based on active method recommended by USEPA (Environmental Protection Agency) 2013 standard where one sample was taken every 6 days (total 21samples). Samples in dusty days (PM10 ≥ 150 µg/m3) were taken when dust storm events occurred (a total of 10 samples).
Collection of airborne fungi samples was carried out at each collection point at 1.5 m above the floor surface and one meter away from an exterior wall using a sample pump for 2 - 5 minutes (Quick Take 30, UK). In the daily run, the pump was set for a flux of 28.3 (L/min) by calibrator (Defender15, UK) (9). After collecting the air samples, the Petri dishes were immediately sent to the laboratory. About 100 (mg/L) of Chloramphenicol was added to the media for culturing fungi to inhibit bacterial growth. Cultures were incubated for 72 hours at room temperature (19).
Plates were inspected 4 days after primary exposure. The direct microscopic examination was performed with Lactophenol Aniline Blue stain. Airborne fungi were identified under optical microscope magnification to assess the colony’s shape and color, vegetative hyphae, sexual and asexual reproductive organs. All fungal concentrations were expressed as CFU per cubic meter of air (20). We used Atlas of Clinically Important Fungi with the aid of a mycologist to identify the fungi (21).
3.3. Statistical Analysis
Statistical analysis was performed by using SPSS software version 16. After Kolmogorov-Smirnov test (P < 0.01) for data normal distribution, Kruskal-Wallis test and Mann-Whitney U test were applied. The values for normalization were evaluated by the Kolmogorov-Smirnov test and the test indicated (P < 0.01) that the results did not fit a normal distribution. Also, Spearman’s correlation coefficient was calculated.
Kruskal-Wallis test was used in case of normal distribution of data to compare samples in different places or seasons. The significance difference between fungi concentration of normal and dusty days was demonstrated using Mann-Whitney U. Spearman’s correlation coefficient, a nonparametric statistical test, was used to show the statistical correlation between meteorological data and fungal concentrations in both normal and dusty conditions. The P value of less than 0.05 was considered statistically significant.
3.4. Measurement of Meteorological Parameters
Relative humidity and temperature at each sampling point were measured by Elcometer model: 319-Standard device. Temperature, pressure, wind velocity, Relative humidity, UV-index, and dew point were obtained from the weather channel (22).
3.5. Report of the Fungi
Colony-forming units per cubic meter of air were calculated as follows (23):
C = Number of colonies
V = Velocity of air flow (L/min)
T = Sampling time (min)
4. Results
4.1. Comparison of Fungi Concentration in Normal and Dust Event Days
Table 1 shows the frequencies of the identified fungi. The results showed that the highest concentrations of fungi in normal and dust events days were related to the genus of Cladosporium, which were 440 and 2,277 (CFU/m3), respectively.
| Fungal | Weather Condition | Mean ± SD | Max |
|---|---|---|---|
| Cladosporium | 1 | 440 ± 628 | 2356 |
| 2 | 2277 ± 3888 | 11779 | |
| Penicillium | 1 | 97 ± 91 | 412 |
| 2 | 49 ± 44 | 141 | |
| Aspergillus flavus | 1 | 68 ± 160 | 1119 |
| 2 | 32 ± 54 | 259 | |
| Aspergillus niger | 1 | 49 ± 56 | 247 |
| 2 | 74 ± 141 | 589 | |
| Yeast | 1 | 18 ± 39 | 271 |
| 2 | 10 ± 24 | 94 | |
| Alternaria | 1 | 14 ± 28 | 177 |
| 2 | 26 ± 31 | 141 | |
| Aspergillus | 1 | 9 ± 24 | 141 |
| 2 | 4 ± 7 | 24 | |
| Ostilago | 1 | 8 ± 26 | 141 |
| 2 | 35 ± 53 | 188 | |
| Drechslera | 1 | 5 ± 16 | 106 |
| 2 | 4 ± 11 | 59 | |
| Fusarium | 1 | 5 ± 14 | 71 |
| 2 | 4 ± 12 | 59 | |
| Aspergillus ochraceus | 1 | 5 ± 12 | 59 |
| 2 | 4 ± 8 | 24 | |
| Rhizopus | 1 | 4 ± 6 | 12 |
| 2 | 3 ± 5 | 12 | |
| Sterile hyphae | 1 | 4 ± 11 | 71 |
| 2 | 12 ± 17 | 59 | |
| Aspergillus fumigatus | 1 | 3 ± 8 | 35 |
| 2 | 2 ± 4 | 12 | |
| Curvularia | 1 | 2 ± 82 | 12 |
| 2 | 2 ± 7 | 35 | |
| Rhodotorula | 1 | 2 ± 47 | 8 |
| 2 | 4 ± 10 | 35 | |
| Stemphylium | 1 | 2 ± 9 | 59 |
| 2 | 2 ± 13 | 71 | |
| Tricosporom | 1 | 1 ± 9 | 71 |
| 2 | 0 ± 0 | 0 | |
| Aspergillus terreus | 1 | 1 ± 8 | 24 |
| 2 | 1 ± 4 | 24 | |
| Monelia | 1 | 1 ± 5 | 35 |
| 2 | 1 ± 3 | 12 | |
| Bipolaris | 1 | 1 ± 4 | 24 |
| 2 | 2 ± 6 | 24 | |
| Mucor | 1 | 0 ± 2 | 14 |
| 2 | 2 ± 11 | 59 | |
| Sporotrichum | 1 | 0 ± 3 | 24 |
| 2 | 0 ± 0 | 0 | |
| Paecilomyces | 1 | 0 ± 2 | 12 |
| 2 | 0 ± 2 | 12 | |
| Acremonium | 1 | 0 ± 1 | 12 |
| 2 | 0 ± 0 | 0 | |
| Trichoderma | 1 | 0 ± 1 | 12 |
| 2 | 0 ± 0 | 0 | |
| Syncephalastrum | 1 | 0 ± 1 | 12 |
| 2 | 0 ± 0 | 0 | |
| Nigrospora | 1 | 0 ± 0 | 0 |
| 2 | 1 ± 3 | 12 | |
| Circenela | 1 | 0 ± 0 | 0 |
| 2 | 0 ± 2 | 12 | |
| Ulocladium | 1 | 0 ± 0 | 0 |
| 2 | 0 ± 2 | 12 |
a1, normal; 2, dusty.
4.2. Fungi Concentrations in Different Seasons
Table 2 shows fungi concentration in the summer and autumn. As can be observed, the highest concentrations of fungi in the summer and autumn events were related to the genus of Cladosporium, which were 239 and 1,733 (CFU/m3), respectively. The results showed that the difference in mean colony distribution of fungi was statistically significant (P < 0.05) in terms of summer and autumn seasons.
| Fungal | Seasons | Mean ± SD | Max |
|---|---|---|---|
| Cladosporium | 1 | 239 ± 290 | 1060 |
| 2 | 1733 ± 3128 | 11779 | |
| Penicillium | 1 | 82 ± 80 | 389 |
| 2 | 82 ± 86 | 412 | |
| Aspergillus flavus | 1 | 88 ± 190 | 1119 |
| 2 | 29 ± 36 | 141 | |
| Aspergillus niger | 1 | 58 ± 101 | 589 |
| 2 | 55 ± 84 | 530 | |
| Yeast | 1 | 21 ± 45 | 271 |
| 2 | 10 ± 23 | 94 | |
| Alternaria | 1 | 11 ± 16 | 59 |
| 2 | 24 ± 36 | 177 | |
| Aspergillus | 1 | 9 ± 19 | 94 |
| 2 | 6 ± 22 | 141 | |
| Ostilago | 1 | 0 ± 0 | 0 |
| 2 | 32 ± 48 | 188 | |
| Drechslera | 1 | 6 ± 17 | 106 |
| 2 | 4 ± 11 | 59 | |
| Fusarium | 1 | 4 ± 12 | 71 |
| 2 | 6 ± 14 | 71 | |
| Aspergillus ochraceus | 1 | 5 ± 11 | 59 |
| 2 | 5 ± 11 | 47 | |
| Rhizopus | 1 | 3 ± 5 | 12 |
| 2 | 5 ± 6 | 12 | |
| Sterile hyphae | 1 | 3 ± 10 | 59 |
| 2 | 9 ± 26 | 71 | |
| Aspergillus fumigatus | 1 | 2 ± 7 | 35 |
| 2 | 3 ± 7 | 35 | |
| Curvularia | 1 | 3 ± 14 | 82 |
| 2 | 1 ± 5 | 35 | |
| Rhodotorula | 1 | 2 ± 7 | 35 |
| 2 | 4 ± 10 | 47 | |
| Stemphylium | 1 | 1 ± 7 | 47 |
| 2 | 3 ± 13 | 71 | |
| Tricosporom | 1 | 2 ± 11 | 71 |
| 2 | 0 ± 2 | 12 | |
| Aspergillus terreus | 1 | 1 ± 4 | 24 |
| 2 | 1 ± 5 | 24 | |
| Monelia | 1 | 0 ± 2 | 12 |
| 2 | 2 ± 6 | 35 | |
| Bipolaris | 1 | 0 ± 0 | 0 |
| 2 | 2 ± 6 | 24 | |
| Mucor | 1 | 0 ± 0 | 0 |
| 2 | 2 ± 9 | 59 | |
| Sporotrichum | 1 | 0 ± 0 | 0 |
| 2 | 0 ± 3 | 24 | |
| Paecilomyces | 1 | 1 ± 2 | 12 |
| 2 | 0 ± 2 | 12 | |
| Acremonium | 1 | 0 ± 2 | 12 |
| 2 | 0 ± 0 | 0 | |
| Trichoderma | 1 | 0 ± 0 | 0 |
| 2 | 0 ± 2 | 12 | |
| Syncephalastrum | 1 | 0 ± 0 | 0 |
| 2 | 0 ± 2 | 12 | |
| Nigrospora | 1 | 0 ± 0 | 0 |
| 2 | 0 ± 2 | 12 | |
| Circenela | 1 | 0 ± 0 | 0 |
| 2 | 0 ± 2 | 12 | |
| Ulocladium | 1 | 0 ± 0 | 0 |
| 2 | 0 ± 2 | 12 |
a1, summer; 2, autumn.
4.3. Relationship Between Concentration of Fungi and Meteorological Parameters
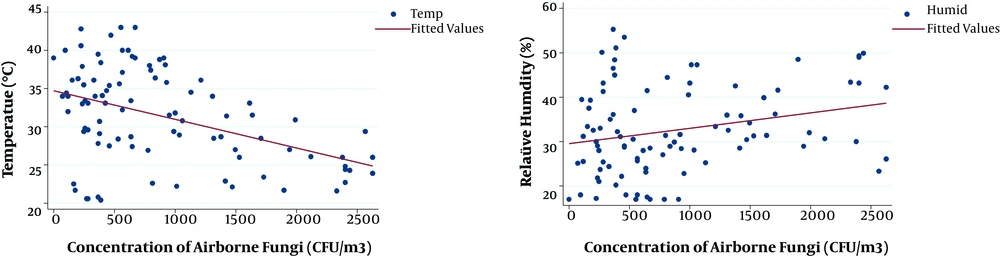
Table 3 shows ambient fungal concentrations have a significant relationship with meteorological parameters (P < 0.05). The Figure 2 shows the relationship between the concentrations of airborne fungi and simultaneously measured meteorological factors.
| Variables | Ambient Fungal Concentrations, CFU/m3 |
|---|---|
| Temperature, °C | |
| Correlation coefficient | -0.357a |
| P valueb | < 0.001 |
| Pressure, mmHg | |
| Correlation coefficient | 0.453a |
| P value | < 0.001 |
| Wind velocity, m/sec | |
| Correlation coefficient | 0.212c |
| P value | 0.042 |
| RH, % | |
| Correlation coefficient | 0.198 |
| P value | 0.050 |
| UV-index | |
| Correlation coefficient | 0.046 |
| P value | 0.664 |
| Dew point, deg | |
| Correlation coefficient | -0.035 |
| P value | 0.742 |
aCorrelation is significant at the 0.01 level (2-tailed).
bSpearman’s rho test was used to examine the relationship between ambient fungal concentrations and meteorological parameters.
cCorrelation is significant at the 0.05 level (2-tailed).
4.4. Relationship Between Stations and Ambient Fungal Concentrations
The Figure 3 shows the distribution of fungal bioaerosols in the three study areas was almost the same, and there was no statistically significant difference.
5. Discussion
Fungi are often in contact with humans and have harmful effects such as infections, allergy, and irritation (10, 18). The complications resulting from fungi differ based on their type and species (18, 24). Therefore, the isolation of fungi from environmental resources is one of the basic principles for their identification and determining their potential role in causing various complications in humans. Fungal spores are seen almost everywhere, but their type and number vary depending on the time of day, season, weather, ambient temperature, humidity, rainfall, geographic location and spore sources (18, 24-26). In the present study, different genus and species of fungi were isolated from Ahvaz ambient air. Filamentous fungi were the most common isolated fungi. The total average of fungal colonies isolated from the study areas was 899 (CFU/m3), while Gami obtained 542 (CFU/m3) in his study. One of the reasons for the presence of fungal spores in Ahvaz air is its location in a hot and dry climate with rainfall less than 250 mm per year.
The present study showed that the number of fungi colonies was higher in autumn than summer due to dust storm events. Also, in this study, the average distribution of fungi colonies in dusty days was higher than that in normal days. The average number of fungi colonies in normal and dusty days was 740 and 1,245 (CFU/m3), respectively. There is a significant difference between the type of fungi in normal and dust conditions (P < 0.05). It can be concluded from the present study that dust storms are one of the most important factors affecting bioaerosol density. This high proportion is also found in Shahsavani et al. study.
According to the obtained data, the total average number of fungal colonies during the study period in the summer and autumn was 539 and 1,229 (CFU/m3), respectively. However, in Mazlomi et al. study conducted in Ilam in 2012, the total number of fungi colony in different seasons was significantly different. In the present study, the total average number of fungal colonies was lower in summer. Thus it can be concluded that by increasing temperature, the number of bioaerosols drops. So the higher the air temperature, the dryer air, and less moisture; therefore, more unfavorable conditions are available for fungi survival.
A total of 6,160 filamentous fungi were observed in the summer and autumn seasons. Cladosporium, Aspergillus flavus, Penicillium and Aspergillus niger were the most prevalent, while the least frequent fungi were Acremonium, Cinnamon, Cephalosporum, and Monelia in summer. On the other hand, in autumn Cladosporium, Penicillium, Osteaglia, and Aspergillus niger were the highest in the number and Aspergillus nidulans, Psilomyces, and Oluclacidium were the lowest. There is no obvious reason for these differences; however, they can be attributed to different types of the sampler, the different type of culture media (fungal growth substrates), different experimental and sampling approaches, as well as geographic location. One reason for the high number of Cladosporium fungus in the summer and autumn could be related to the pigments of the fungi. The presence of melanin pigments in the structure of cell membrane in this fungus makes its adaptation to the environment and natural selection possible. In fact, the melanin pigment protects the organism from destructive effects of the sun’s ultraviolet radiation. In our study area, Ahvaz, the highest radiation occurs in most months of the year. Owing to their inherent thermophilicity, Aspergillus and Penicillium prefer higher temperatures for growth; therefore, it is obvious that they proliferate in the summer. Predominant genera of the identified fungi in dust storms were Cladosporium, Alternaria, Penicillium, Aspergillus, Rhizopus, and Mucor. On the other hand, Cladosporium, Penicillium, Aspergillus, Alternaria, Acremonium, Mucor, and Rhizopus were the predominant genera of fungi in normal days. According to the results of the present study, the genera of fungi extracted in dust storm conditions were similar to those of other studies conducted in other parts of the world.
The highest number of fungi in Ahvaz air during the study period belonged to Cladosporium with a mean value of 440 (CFU/m3) for normal days and 2,277 (CFU/m3) for dusty days. It can be concluded that due to the spore size of Cladosporium fungus, larger spores from the sample are more precipitated and absorbed on the culture medium. Shahsavani et al. reported that the highest frequency of fungi in Ahvaz air was related to Cladosporium, Penicillium, Alternaria, and Aspergillus in normal conditions while in dusty conditions, it belonged to Rhizopus, Cladosporium, and Penicillium (27). Studies conducted in Tehran and Isfahan reported the most frequent isolated fungi were Penicillium, Aspergillus, Alternaria, and yeasts (18). All identified fungal species were able to form spores, which would protect these species against environmental changes. Therefore, the prevalence of these species and genus is attributed to their metabolic ability, which preserves their distribution and survival in adverse environmental conditions such as ultraviolet radiation, lack of nutrients or high temperatures (26). The type and genus of isolated fungi in dusty conditions were influenced by dust source and distance. Considering Ahvaz, which is in close proximity to a neighboring country, Iraq (28), some of the dusty storms entering the city come from deserts of Iraq, Saudi Arabia, Kuwait, and North Africa (29-31). Most studies suggest that the most common isolated fungi from soil samples of some of these countries are Aspergillus, Fusarium, and Penicillium (32). Consistent with our results, these studies show that dominant fungal species dust deposits are Cladosporium, Alternaria, and Aspergillus (28, 29, 33-35).
5.1. Conclusions
Our findings show that the total fungi concentration was found to be 6,160 (CFU/m3) in Ahvaz. Maximum fungi concentration was found to be on average 239 (CFU/m3) in summer. In general, the emission of fungal bioaerosols was high, suggesting hazardous levels of bioaerosol. Cladosporium, Penicillium, Aspergillus niger, and Aspergillus flavus were the most frequently observed fungus types in Ahvaz ambient air. One possible reason for this condition is that these genera are resistant in unfavorable conditions. This study notes that the suggested numerical guidelines for “acceptable” levels of “total airborne fungi” vary and that widespread agreement on their relevance is lacking. Irrespective of this variety and disagreement, the authors fail to consider any ecological groupings of fungi and continue to emphasize total airborne fungi by concluding that since levels in outdoor air frequently exceed 500 (CFU/m3), the numerical standard is too low.