1. Background
Organic compounds are present in water supplies, causing different problems in water treatment processes. Humic and fulvic acids comprise the largest group of organic materials in surface waters supplies. Humic acids account for 60-90% of natural organic compounds in water (1-3). They are not toxic in nature, but create secondary problems in water treatment, such as change of color, taste, and odor, increased erosion of pipe structures, increased movement of heavy metals, and decreased efficiency of water treatment processes (4, 5).
The adsorption process, as a simple and economic method, is used for the removal of organic pollutant compounds (6, 7). Use of activated carbon, membrane processes, and advanced coagulation are among common methods for the removal of trihalomethanes. In this regard, a study by Omri and colleagues from Taiwan evaluated the removal of humic acid by activated carbon derived from almond shell, as a method for phosphoric acid removal from industrial wastewater. The results showed that this method is both economic and effective for the removal of humic acid from phosphoric acid solution (8). Activated carbon has high porosity and adsorption capacity, although it becomes saturated after a while; therefore, this adsorbent should be regenerated.
In recent years, new techniques for regeneration of activated carbon have been developed. Biological regeneration involves bacterial reactions and has some advantages, such as cost-effectiveness and low carbon waste. However, this method has not been applied at the industrial scale (9). On the other hand, chemical regeneration has some advantages, such as very low carbon waste, which reaches almost zero in catalytic regeneration and extraction using supercritical fluids. Chemical regeneration usually occurs at ambient temperature; therefore, control of temperature is not very important (10, 11).
Use of thermal methods requires great energy. In these methods, a temperature range of 800-850°C is usually set (12, 13). In pressure regeneration, atomic charge is usually applied (e.g., CO2) (14). Ultrasonic regeneration has many advantages, such as removal of unwanted pollutants, decomposition of toxic organics, and most importantly saving energy, which increases the popularity of this method (15). Feng et al. reported that organic and mineral particles on the adsorbent can be successfully released through the sonication process (16). Moreover, Zhang et al. performed a study on regeneration of activated carbon with the ultrasound process in water treatment; the results showed the effectiveness of this method (17). Considering the diversity of ultrasonic regeneration advantages, such as lower energy consumption, simpler equipment, lower carbon loss, and higher recovery of valuable substances, we aimed to survey the ultrasonic regeneration of saturated activated carbon with humic acid.
2. Methods
This experimental study examined the function of activated carbon in humic acid removal from water solutions. In order to regenerate saturated activated carbon, the ultrasonic process was applied. Activated carbon used in this study was purchased from the Research Center of Petroleum Industry. All chemicals in this study, including hydrogen chloride, sodium hydroxide, and humic acid (purity, 55%), were purchased from Merck Company. A UV-visible spectrophotometer was used to analyze humic acid samples at 254-nm wavelength. Also, for regeneration of the adsorbent, an ultrasonic device (Elmasonic E30H) was employed at a frequency of 37 kHz and power of 240 W.
2.1. Adsorption Experiments
The stock solution of humic acid was collected by dissolution of humic acid powder (humic acid sodium salt, 45% priority) in deionized water. Then, solutions with the initial concentrations of 2, 5, and 10 mg/L were prepared via dilution. Various parameters, such as initial concentration of humic acid, pH (3, 5, 7, 9, and 11), contact time (5, 10, 15, 30, and 45 minutes), and adsorbent dosage (0.1, 0.2, and 0.3 g/L) were evaluated. The following equation was used for calculation of the adsorption capacity (18):
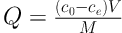
where Q denotes the amount of humic acid adsorbed by activated carbon (mg/g); C0 is the initial humic acid concentration (mg/L); Ce is the equilibrium concentration of humic acid (mg/L); V is the initial solution volume (L), and M is the activated carbon dosage (g).
2.2. Regeneration Experiments
In order to saturate the adsorbent, 1 g of activated carbon was added to a 250-ml Erlenmeyer flask, containing 500 mg/L of humic acid solution. Then, the flask was placed in a shaker (240 rpm) for 120 minutes to make sure that the adsorbent is fully saturated. At this stage, various parameters, such as pH, regeneration time, and number of regeneration cycles, were evaluated.
3. Results
3.1. Adsorbent Characterization
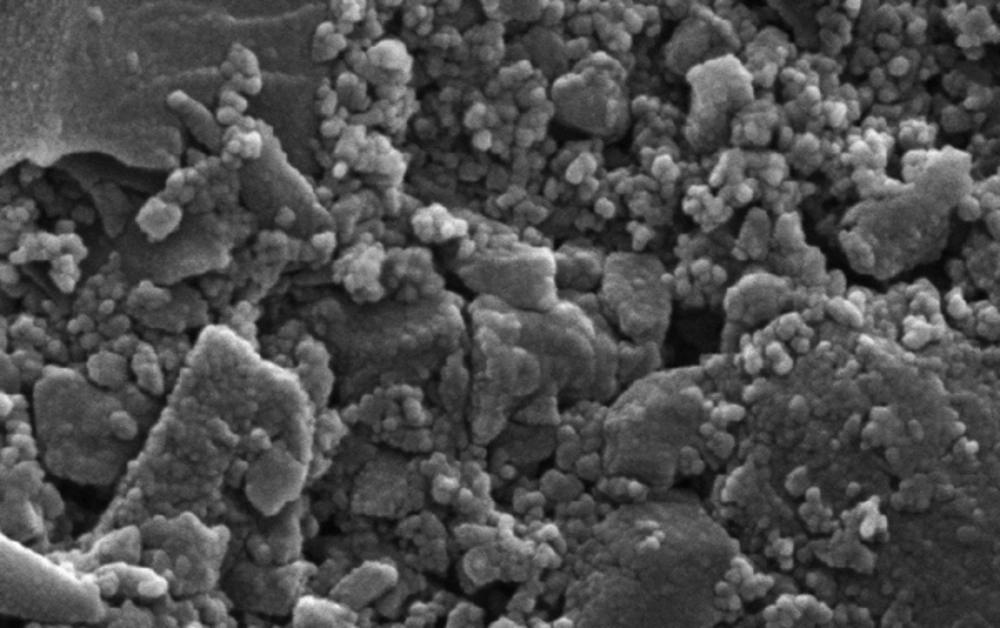
For accurate measurement of the diameter of activated carbon, scanning electron microscopy (SEM) was applied. This technique provides information about the surface morphology. Figure 1 presents the SEM micrographs of activated carbon.
3.2. Effect of Solution pH on Humic Acid Adsorption
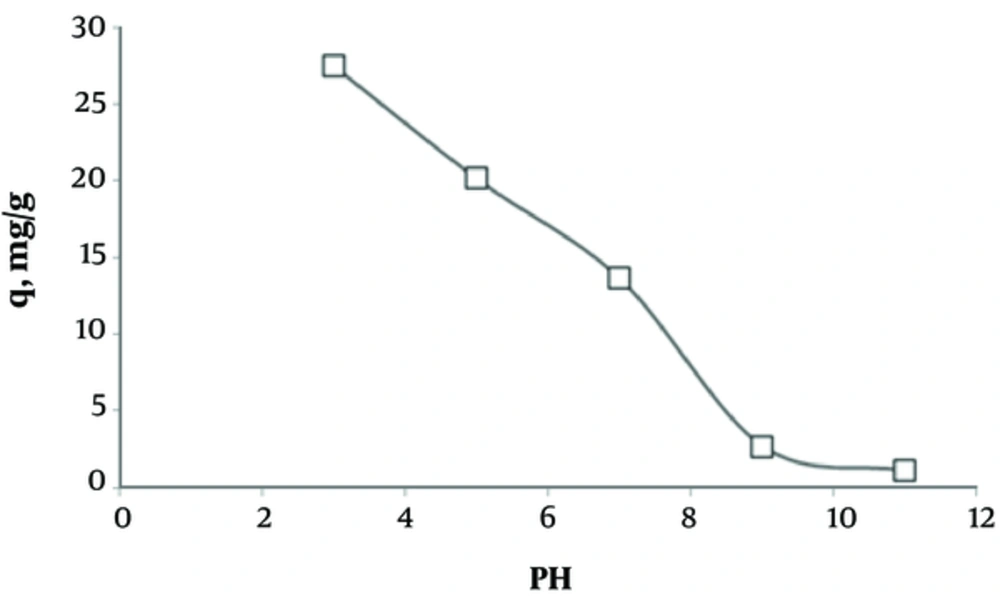
The results regarding the effects of pH on humic acid adsorption are presented in Figure 2. According to this figure, by increasing the solution pH from 3 to 11, the adsorption capacity decreased.
3.3. Effect of Adsorbent Dosage on Humic Acid Adsorption
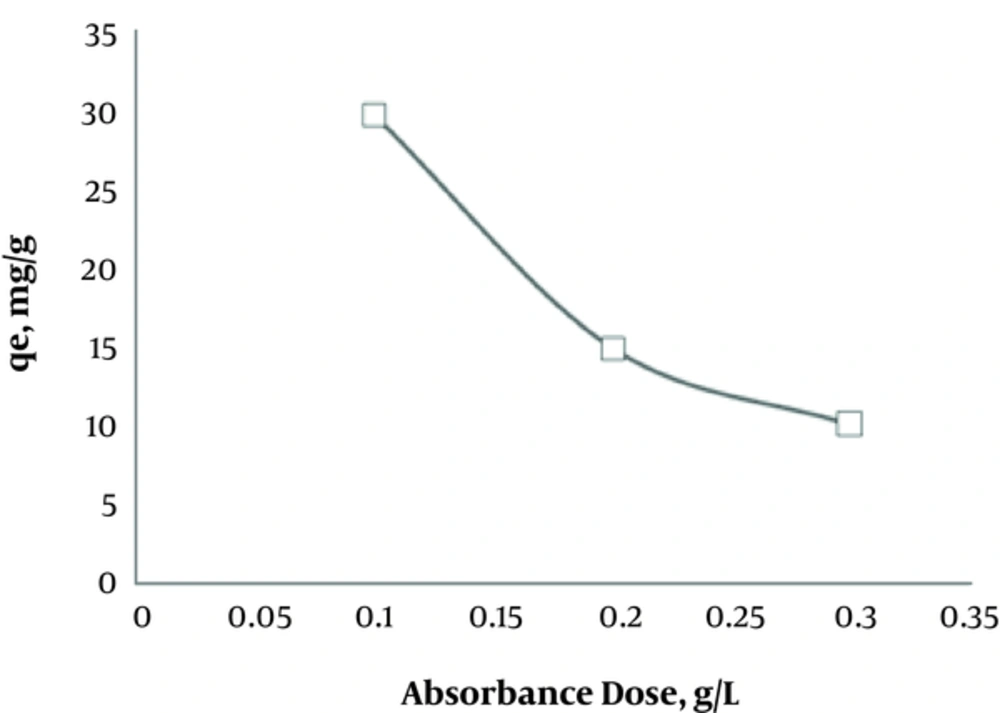
The effects of various activated carbon doses (0.1, 0.2, and 0.3 g/L) on the removal of humic acid are shown in Figure 3. The optimized concentration of activated carbon was determined as 0.01 g/L.
3.4. Effects of Contact Time and Initial Concentration on the Removal of Humic Acid
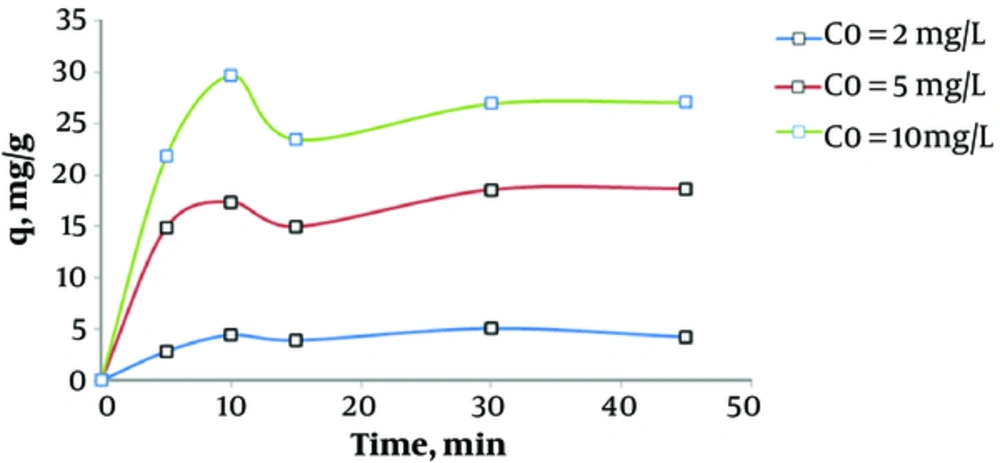
The results presented in Figure 4 indicate that at humic acid concentrations of 2, 5, and 10 mg/L, the adsorption capacities were 4.39, 17.37, and 29.73 mg/L, respectively, as the contact time reached 10 minutes.
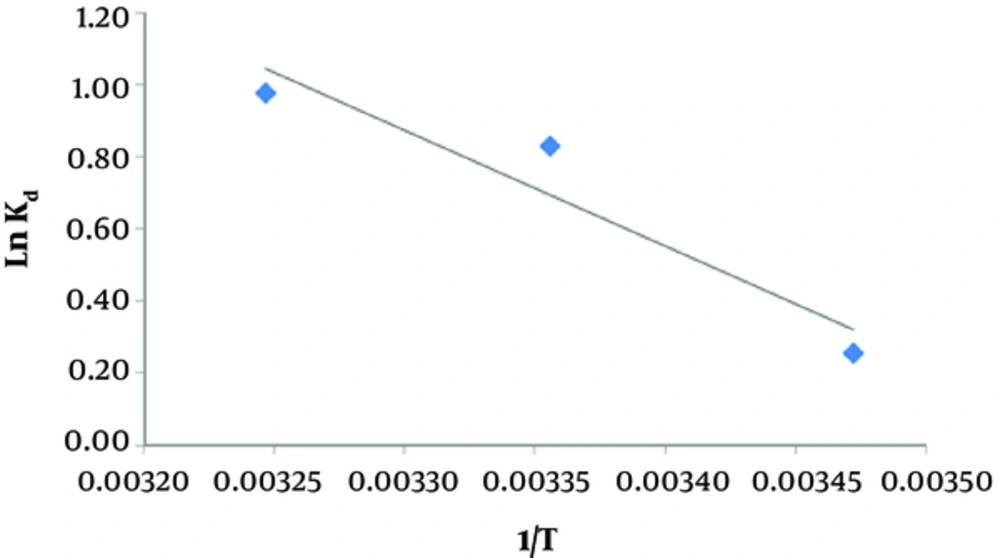
3.5. Effect of Temperature and Evaluation of Thermodynamic Parameters
As presented in Figure 5 and Table 1, increasing the temperature can elevate the adsorption of humic acid on activated carbon.
| T, K | C0 | Ce | Activated carbon | |||
|---|---|---|---|---|---|---|
| mg/L | mg/L | ∆G (kJ/mol) | ∆H (kJ/mol) | ∆S (J/mol K) | R2 | |
| 288 | 10 | 8.85 | -0.62 | 26.65 | 95.20 | 0.91 |
| 298 | 10 | 8.13 | -2.05 | |||
| 308 | 10 | 7.90 | -2.50 | |||
Thermodynamic Parameters of the Adsorption Process
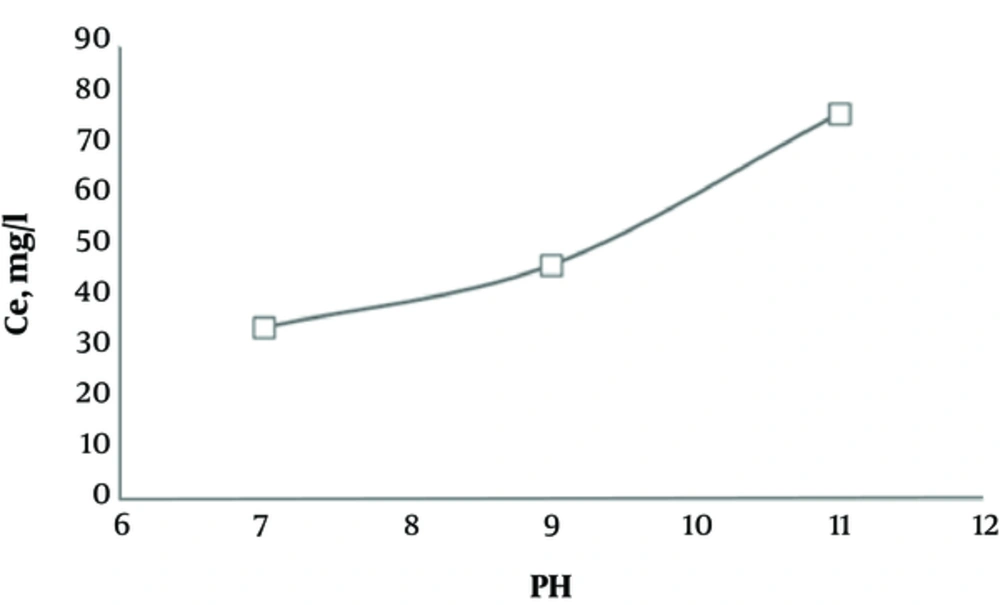
3.6. Effect of pH on Ultrasonic Regeneration of Activated Carbon Saturated with Humic Acid
According to Figure 6, by increasing pH, regeneration of activated carbon saturated with humic acid increased; the concentration of extracted humic acid from activated carbon reached 76.4 mg/L at pH of 11.
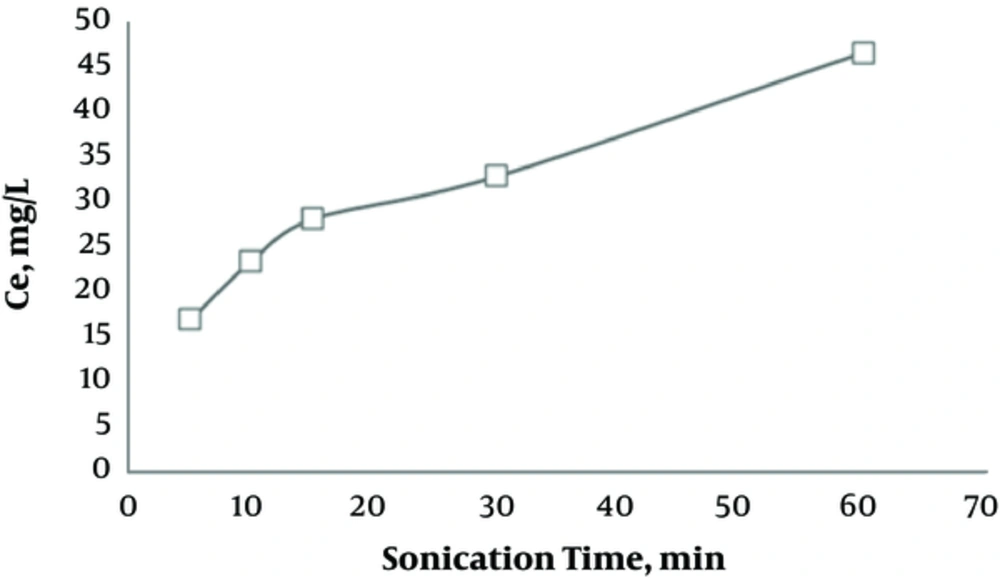
3.7. Effect of Sonication Time on Regeneration of Activated Carbon Saturated with Humic Acid
Figure 7 shows that by increasing the sonication time, the concentration of extracted humic acid from activated carbon increased.
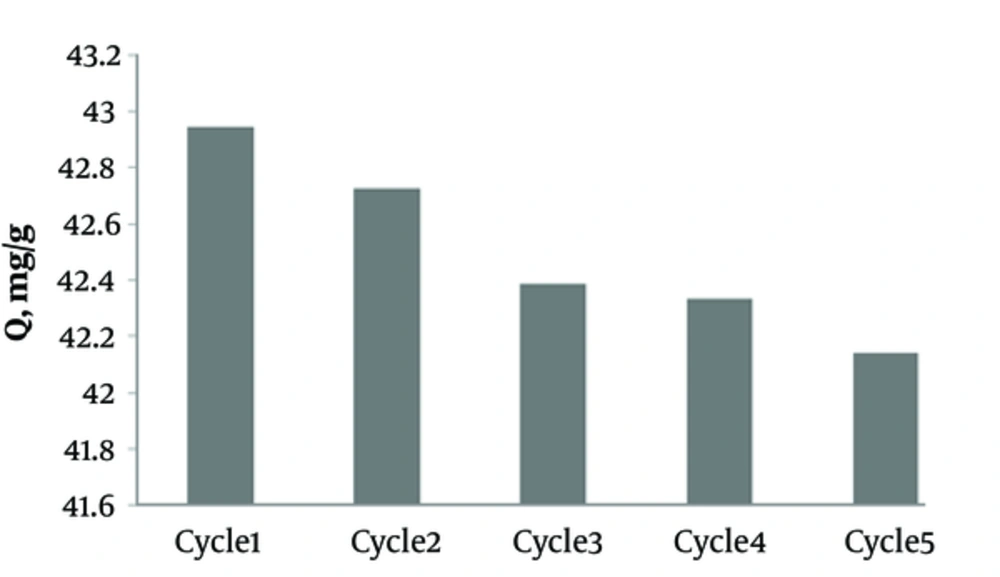
3.8. Effects of Regeneration Cycles of Activated Carbon Saturated with Humic Acid
The results of regeneration cycles of activated carbon saturated with humic acid are shown in Figure 8.
The results showed that after each cycle of regeneration, removal of humic acid by activated carbon decreased slightly. Therefore, the adsorption capacity of activated carbon after five cycles of ultrasound regeneration (frequency, 37 kHz) decreased from 42.94 to 42.14 mg/g.
4. Discussion
The present results showed that by increasing pH, the adsorption capacities decrease. Humic acid shows high solubility at acidic pH, which affects its adsorption. Also, pH influences the surface density and adherence of particles. OH+ and OH- are two important ions in the removal process and are considered as surface charge-determining ions (19, 20). In addition, as pH increases, the size of humic acid molecules is prone to change from spherical to linear, leading to the reduced adsorption of humic acid onto activated carbon at higher pH; the results of this study are in accordance with previous research (21, 22).
According to Figure 3, as the adsorbent dosage increases, the adsorption capacity of activated carbon decreases; this is because of the active surface of the adsorbent and dynamic factors, such as increased extent of collision and free bands on the adsorbent. Based on the findings, increasing the adsorbent dosage caused an increase in the distribution of different adsorption sites and resulted in the decreased removal of humic acid. On the other hand, under such conditions, a competition is initiated among pollutant molecules to occupy the empty surface of the adsorbent. Consequently, the whole surface of the adsorbent is not used, and the adsorbent capacity cannot be efficiently utilized (21). The results of this study are in accordance with the findings reported by Moriguchi et al., who used modified metals with silica nanoparticles to remove humic acid (23).
According to Figure 4, adsorption decreases as the contact time advances. During the first minutes, maximum free surface is available for the adsorbent. The results showed that adsorption of humic acid is a function of its initial concentration. In fact, adsorption capacity improves by increasing the initial concentration of humic acid. These results are in accordance with a study by Wang et al. in 2006 (24). In this study, maximum adsorption occurred within the first ten minutes, and as the contact time increased, the adsorption capacity reached a steady state. These changes can be probably explained by the fact that within the first minutes of contact, most of the adsorbent surface is empty, and changes of pollutant concentration increase as the pollutant is in the liquid phase. As the contact time increases, less adsorbent surface is vacant, which in turn decreases the velocity of changes in the liquid pollutant and consequently reduces adsorption (25).
On the other hand, the repulsive force between particles on the surface of the adsorbent increases by time, while the velocity of adsorption decreases (26). Lu and Su studied the adsorption of natural organic materials from aqueous solutions on carbon nanotubes. They found that adsorption improved as the initial concentration of organic materials increased, while adsorption decreased by increasing pH (9).
According to Table 1, as the temperature increases, the removal efficiency of humic acid improves. Also, the enthalpy value is positive, which shows that the adsorption process of humic acid onto activated carbon is endothermic and probably a physical adsorption process. The positive value of entropy shows that the degree of freedom increases at the solid-liquid interface during humic acid adsorption onto activated carbon. The results of this study are in accordance with a study by Zolfikar on the effects of temperature on humic acid removal (27). In other studies, researchers have reported similar findings (28). In fact, increasing the temperature improves the distribution of humic acid molecules in the external layers and internal pores of the adsorbent (29).
According to Figure 6, by increasing pH, the amount of humic acid introduced to distilled water from the adsorbent increases. In addition, at pH of 11, concentration of the extracted humic acid from activated carbon reached 76.4 mg/L. Therefore, regeneration of saturated activated carbon with humic acid occurred more efficiently at higher pH ranges. Rege and colleagues reported the same results on regeneration of polymeric saturated carbon with phenol, using the ultrasonic process (11, 30).
According to Figure 7, as the sonication time advances, the humic acid concentration extracted from activated carbon increases, as well. In this regard, Hamdaoui et al. studied the effects of ultrasonic process on adsorption of activated carbon and reported an improvement in regeneration efficiency as sonication time increases (15, 31). The impact of the frequency of regeneration cycles on adsorption capacity is presented in Figure 8. The results showed that after each cycle of saturation and regeneration, the adsorption capacity decreased. As in the first phase of saturation, the adsorption capacity was 42.94 mg/g, while it reduced to 42.14 mg/g after a regeneration cycle. The reduced adsorption capacity might be related to the deposition of decomposed residues in activated carbon pores, which blocked carbon porosity (11, 13, 15, 30).
4.1. Conclusion
In this study, activated carbon was used as an adsorbent to remove humic acid from water solutions; also, the ultrasonic process was applied at a frequency of 37 kHz to regenerate saturated activated carbon with humic acid. The maximum removal of humic acid occurred at pH of three, and maximum regeneration efficiency was reported at pH of 11. Generally, the results of this study revealed that activated carbon could be a proper adsorbent to remove humic acid from water solutions. Also, the ultrasonic process showed great capacity to regenerate activated carbon and recycle it for humic acid removal.