1. Background
Diabetes mellitus is a chronic disease caused by the lack of insulin secretion or decreased insulin sensitivity in body cells (1). Over time, it can cause damage to various organs of the body such as the heart and kidneys (2). Recent research suggests a link between Alzheimer’s disease (AD) and diabetes so that increased blood glucose may be the cause of AD and people with diabetes are more at risk of developing AD than healthy people (3). Alzheimer’s disease is a neurodegenerative disorder characterized by the lack of memory and perception and this type of dementia is involved in the daily function and daily life of the individual (4).
Some areas in the brain such as the hippocampus, amygdala, and prefrontal cortex are the areas of learning (5). The distribution of neurotrophic factors is reported in various regions of the brain, especially the hippocampus, which is responsible for memory and learning and plays a key role in maintaining the health of neuronal cells (6). In pathological conditions, the level of neurotrophic factors changes in the brain and the activation of signaling pathways decreases. Due to the decrease in the level of the nerve growth factor (NGF) protein and its receptor, retrograde axonal transport is reduced (7). In addition, NGF can cause the maturation of young neural cells and it promotes the release of factors in the brain which destroy free radicals and strengthen brain-derived neurotrophic factor (BDNF) (8).
Research has shown that neurogenesis declines more in people with diabetes than in healthy people. Since the discovery of this relationship is related to recent years, a few studies have been done, especially on the effect of exercise training on reducing the AD in people with diabetes. However, most research has been done merely on AD and a few studies have focused on whether people with diabetes are exposed to reduced neurogenesis and hence, the AD. Considering, there are different opinions about the timing of neurogenesis reduction in people with diabetes and there has been little research on the effects of exercise training on reducing neurogenesis in diabetics, we made an attempt to investigate the NGF level in rats several weeks after injecting diabetes and doing long-term aerobic activity to survey when the level of NGF decreases.
2. Objectives
Therefore, this study aimed to survey the rate of NGF decrease several weeks after injecting diabetes in rats. Moreover, we examined whether several weeks of long-term aerobic activity increase the rate of NGF in diabetic rats.
3. Methods
3.1. Study Design
The subjects consisted of 84 rats. The environment temperature was 21 ± 2°C, the cycle of light and dark was 12 h/12 h, and humidity was 60% ± 5%.
Diabetes induction in our research, diabetes was induced by a combination of nicotine amide and streptozotocin (STZ) (9). Nicotine amide was injected initially; then, STZ was injected intraperitoneally after 15 min (10). After five days, fast blood glucose was measured and its level of 126 - 400 mg/dL indicated diabetes (10).
3.2. Experimental Design
The study had four groups of 21 rats. At the end of each step of the training protocol, we eliminated seven rats from each group; thus each of the groups received 21 rats. Subjects practiced for 12 weeks in three sessions per week. The practice load was planned by the principle of over loading. In the first week of training, the speed of practice was 12 m/min.
After the first week, we increased the training speed by one meter per minute every week. From the second to 10th weeks, the practice time increased by 2.20 min every day regularly. In the end, the training period went from 20 to 80 min. The duration of training was kept constant for the last two weeks; the duration and intensity of exercise remained constant so that the possible changes were the result of the adjustment to exercise rather than the response to the intensity of training in the last sessions. The intensity of activity was determined by speed (11). The period of adaptation to activity in groups was one week, 5 min per day at a speed of 5 to 7 m/min. Meanwhile, the heating and cooling at the beginning and the end of the protocol were 3 to 5 min at a speed of 5 - 7 m/min (Figure 1).
Analytical methods the level of NGF was measured in the hippocampal tissue of rats by the ELISA method according to the manufacturer’s instructions (Zellbio Germany). The dispersion coefficient and sensitivity of this method were 6.9% and 2.5 ng/L, respectively. Animals were sacrificed by injecting intraperitoneally with a combination of ketamine (40 - 60 mg/kg body weight) and xylazine (5 - 15 mg/kg body weight).
3.3. Statistical Analysis
The data were analyzed using repeated-measures ANOVA and the pairs were compared with the Bonferroni correction.
4. Results
There was no difference in baseline weight at all stages (P = 0.651, 0.584, 0.406). Nevertheless, the final weight of rats at the end of each stage was different (P = 0.001) (Table 1). The results of the Mauchly’s test were not significant for NGF (P > 0.05) (Table 2). The results of repeated measures ANOVA showed significant differences in NGF in different steps (Table 3). The results of the Bonferroni test showed significant differences between all weeks of training, while the eighth and 12th weeks did not differ significantly (Table 4).
| HCG | HPG | DCG | DPG | P Value | |
|---|---|---|---|---|---|
| First step | |||||
| BW | |||||
| Baseline | 277.2 ± 35.9 | 271.5 ± 21.9 | 282.6 ± 22.8 | 267 ± 24.4 | 0.751 |
| After step 1 | 281.4 ± 33.4 | 272.9 ± 33.4 | 215.90 ± 20.5 | 251 ± 30.2 | 0.002 |
| FG | |||||
| Baseline | 87.1 ± 9.8 | 92.5 ± 3.7 | 367.7 ± 18.9 | 369.3 ± 43.5 | 0.002 |
| Behind step 1 | 86.3 ± 9.5 | 84.7 ± 5.9 | 350.2 ± 100.8 | 203.5 ± 111.8 | 0.002 |
| Second step | |||||
| BW | |||||
| Baseline | 276.6 ± 27.1 | 271.7 ± 15.3 | 287 ± 16.8 | 265.4 ± 18.1 | 0.684 |
| Behind step 2 | 293.4 ± 21.1 | 288.7 ± 16.2 | 214.2 ± 44.7 | 252.4 ± 28.4 | 0.002 |
| FG | |||||
| Baseline | 89.6 ± 4.6 | 88.7 ± 3.4 | 353.2 ± 53.5 | 352 ± 52.89 | 0.003 |
| Behind step 2 | 91.5 ± 5 | 72.2 ± 11.3 | 419.6 ± 97.5 | 213.4 ± 97.7 | 0.003 |
| Third step | |||||
| BW | |||||
| Baseline | 301 ± 13.8 | 282.2 ± 11 | 287.4 ± 21.1 | 279.7 ± 41 | 0.306 |
| Behind step 3 | 302.8 ± 15.9 | 315.8 ± 15.4 | 213.2 ± 44.3 | 293.5 ± 44.4 | 0.003 |
| FG | |||||
| Baseline | 93.1 ± 9.9 | 92.2 ± 6.7 | 332.5 ± 61 | 331.2 ± 56.2 | 0.002 |
| Behind step 3 | 92.9 ± 8.5 | 78.8 ± 9.7 | 455.1 ± 115.8 | 91.4 ± 16.1 | 0.003 |
Abbreviations: BW, body weight; DCG, diabetic control group; DPG, diabetic practice group; FG, fasting glucose; HCG, healthy control group; HPG, healthy practice group.
aValues are expressed as mean ± SD.
| Within Subjects Effect | Mauchly’s W | Approx. Chi-Square | DF | Sig. | Epsilon | ||
|---|---|---|---|---|---|---|---|
| Greenhouse-Geisser | Huynh-Feldt | Lower-Bound | |||||
| Time | 0.871 | 3.175 | 2 | 0.204 | 0.886 | 1.001 | 0.550 |
aMeasure: NGF.
| Source | Type III Sum of Squares | DF | Mean Square | F | Sig. | Partial Eta Squared |
|---|---|---|---|---|---|---|
| Time | ||||||
| Sphericity assumed | 3108.712 | 2 | 1554.356 | 90.312 | 0.001 | 0.790 |
| Greenhouse-geisser | 3108.712 | 1.772 | 1754.797 | 90.312 | 0.001 | 0.790 |
| Time × group | ||||||
| Sphericity assumed | 1674.084 | 6 | 279.014 | 16.211 | 0.001 | 0.670 |
| Greenhouse-geisser | 1674.084 | 5.315 | 314.994 | 16.211 | 0.001 | 0.670 |
aMeasure: NGF.
aThe mean difference is significant at the 0.05 level.
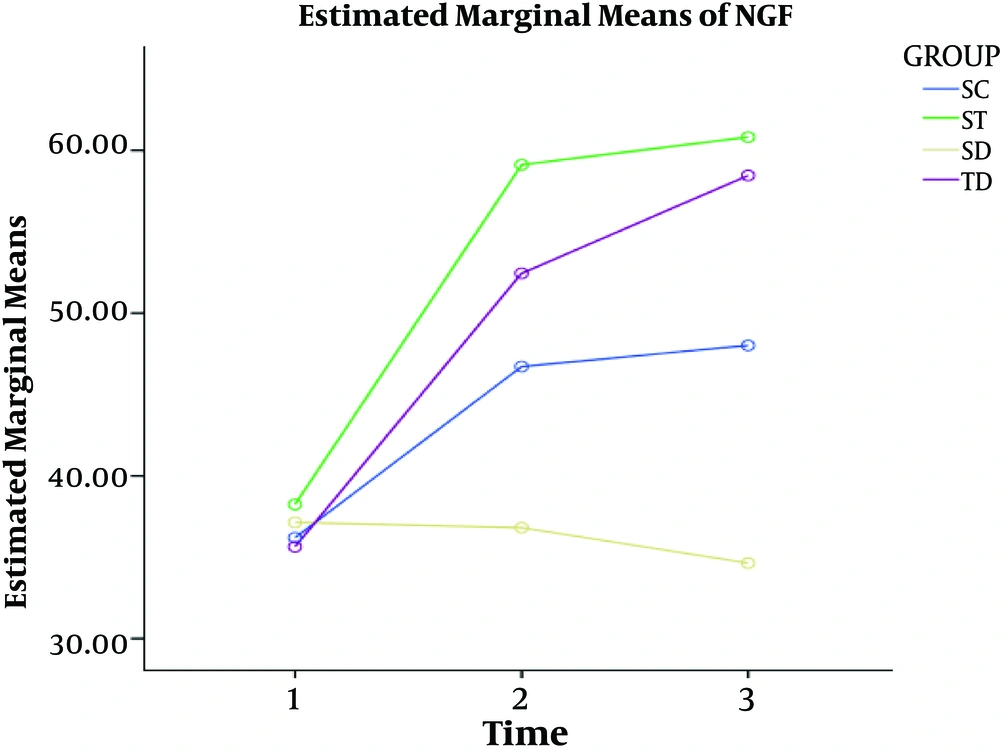
In all three different time periods, the levels of NGF were lower in healthy control and diabetic control groups than in healthy practice and diabetic practice groups. Over time, the level of NGF decreased and long-term aerobic activity could increase the level of NGF (Figure 2).
5. Discussion
The findings did not show significant changes in NGF in diabetic rats during the first four weeks of the study (P = 0.860). As a result, diabetes did not appear to have a significant effect on the growth factor in the hippocampus of diabetic rats in the short-term (four weeks). This finding was contradictory to some studies (7, 12-17) and similar to a previous study (18). The ineffectiveness of diabetes in the hippocampus was possibly due to the short duration of research (four weeks).
Other findings indicated a significant change in the level of NGF in eight and 12 weeks (P = 0.001) so that the level of NGF in HC and DC groups groups reduced eight and 12 weeks after diabetes. This variable showed a significant increase after eight and 12 weeks of aerobic training. This result showed the effect of exercise training on increasing the NGF in diabetic subjects. These findings are similar to previous findings (7, 8, 13, 16, 19) and contradictory to some studies (5, 14, 15, 17).
The findings indicated a significant change in the NGF after all steps of training so that four weeks of aerobic training had a significant difference with eight and 12 weeks of training (P = 0.001). This is while there was no significant difference between eight and 12 weeks of training (P = 0.198). These results are similar to some studies (7, 8, 13, 16, 19).
The NGF signaling is independent of microtubules by activating two different secondary messaging systems (20, 21): one of the involved messaging systems is the MAPK cascade, which functions through Erk phosphorylation, while the other messaging system involves NGF stimulation through activation of the PI3-K/Akt signaling pathway.
Williams et al. (22) observed some ERK and AKT in the hippocampus of aging, NGF-injected rats; here, the phosphorylated ERK level much increased but the level of Akt remained intact. Researchers have proven that these two various pathways overlap. Thus, it seems to improve cognitive performance by the retrograde transport is accelerated with PI3-K/Akt signaling pathway or MAPK cascade (22).
The PI3-K/Akt pathway interferes with diabetes mellitus. reduction in the retrograde transport of neurotrophins and regular exercise does not disrupt the PI3-K activity, but it can activate MAPK (23). Shen et al. (24) showed that rats with regular physical activity had CREB and phosphorylated MAPK/Erk in their hippocampus. In this study, the levels of p-Erk1/2 were higher in the diabetic exercise group than in the diabetic control group. Besides, the CREB transcription factor was phosphorylated (24). The MAPK/Erk is a transcription factor affecting neuronal plasticity and learning in the hippocampus and it is effective in long-term potentiation (LTP) (25-27).
The NGF activation by ERK during LTP can affect cholinergic neurons, cholinergic neurotransmitters, the hippocampus, and the cerebral cortex (28-30). However, the function of cholinergic neurons is impaired in the elderly and patients with pathologic diseases, and ERK function is eliminated (31). This condition reduces the retrograde transport of NGF because the endocytosis of NGF-tyrosine kinases A (Trk A) is dependent on the ERK activity (32).
In general, the activation of the PI3K/AKT signaling pathway, the MAPK cascade by Erk decreased and reduces the retrograde transport of neurotrophins, which reduces NGF as a sign of AD, over time in the brain of diabetic subjects, and Exercise training induce phosphorylation of CREB as well as phosphorylation of MAPK/Erk in the hippocampus of rats (12).
A limitation of the study was that the basic physiological capacity of rats (heart rate, lactic acid level, VO2max, etc.) was not measured.
It is suggested that catecholamines and Insulin-like growth factor 1 (IGF-1) be measured to determine their relationships with exercise training.
5.1. Conclusions
We concluded that the more time you have diabetes, the lower your brain NGF levels will be. Therefore, the long-term aerobic activity could increase the level of this variable.