1. Context
Ketone bodies (KBs), characterized as lipid particles, water-soluble formed inside the liver tissue, can be utilized as a secondary source of energy if glucose is not able to provide enough energy. They show possessing the potential to cross the blood-brain barrier (BBB) and the placental barrier. Acetone, beta-hydroxybutyrate, and acetoacetate are well-known ketone bodies which were generated during the absence of glucose or when the body cannot utilize it as energy in type 1 diabetes (1). Indeed, KBs should not be considered as a simple energy molecule required during synthesis of membrane in physiological development (2). They are formed by the breakdown of fatty acids during a low glucose intake or under a ketogenic diet (3). In fact, KBs are produced during a ketogenic diet or due to glucose starvation, which can influence many metabolic and regulatory enzymes, and subsequently substrates result in a neuroprotective, anti-oxidant, anti-inflammatory, anti-seizure, hyper excitability as well as synchrony in neurons (4). A ketogenic diet includes low carbohydrate- protein and high-fat meals consumption leading towards a starved state. KBs are considered the more efficient alternative energy source in the brain and mitochondrial metabolism. It has been proved that the motor functions could be improved by ketogenic diet or medium length chain glyceride diet affected diseases, i.e., Alzheimer’s disease, motor dysfunction, epilepsy, Parkinson disease, metabolic syndromes, glycogen storage disease, Spinal cord injury and Autism in the rat models (5). In this line, extrahepatic tissues are also able to oxidize KBs, contributing to efficient energy metabolism (6). Recent studies on the regulation of extracted KBs from hepatocytes or perfused liver and rat models in fed to starve state, showed that when the KBs concentration exceeds from normal levels, for example, in diabetes type 1, leads to a pathologic state known as ketoacidosis. The genes which generally play a key role in modulated KBs formation and utilization include Hmgcs2, CPT1A, FABP, LCAD, MCAD, and MCT1, also transcription factors such as SREBF1, PPARG, CEBPA, and PPARα. Currently, the improvement of neurological efficiency via KBs in a regulatory manner with specific diets such as ketogenic diet and its utilization for treating certain neurological disorders and cancer seems to be an interesting issue to focus. Overall, KBs, as an alternative energy source for survival during food deprivation, show the potential to help improving body control and coordination as well as the treatment of multiple diseases.
2. Brain Metabolic Functions With/Without Ketone Bodies and Its Effect on Other Metabolic Processes
Glucose is generally utilized as the main source of fuel, broken down subsequently during the glycolysis pathway (7), which transported with the glucose transporters (GLUT), including different isoforms as well as kinetic constants inside the brain (8). Once glucose enters into the neuronal cytosol, is able to easily participate in the different metabolic pathways comprising glycolysis, tricarboxylic acid (TCA) cycle and electron transport chain (oxidative/non-oxidative phosphorylation), the pentose phosphate or hexose monophosphate shunt pathway (PPP) and synthesis of glycogen (9). During glycolysis, glucose is oxidized and converted into the two molecules of pyruvate by phosphofructokinase-1, (hexokinase (HK), pyruvate kinase, which are greatly expressed inside the neuronal cytoplasm (10). In this context, the TCA (tricarboxylic acid) cycle plays a very important role in the formation of substrates required for electron transport chain and, ultimately Adenosine Triphosphate (ATP) generation inside the mitochondria (11). Given that the brain extremely needs to the glucose-derived energy, it is used primarily in the brain for energy production, and the neurons use nearly 70% of the ATP in the brain. This energy necessity can decrease during neurodegenerative disorders, which decrease glucose utilization/uptake and show a decrease in mitochondrial activity which results in a decreased ATP formation (12). During a failure in the kinetics of foods, further calories required to maintain normal functions of body. After a few days under a starved state, the body begins to generate KBs as a main source for producing energy. The brain tissue is capable of deriving two-third of its metabolic processes from ketone oxidation (13). In fact, KBs enter the brain tissue through mono-carboxylate transporters (MCT) with the higher rates of expression and activity in the brain of new-born than the matured rats. Beta-hydroxybutyrate (BHB) and acetoacetate are absorbed by the brain and used proficiently as substrates for energy production, in protein and lipid bio-formation, which are the most important ways required in the dynamic brain development. It has also been found that KBs about 30% - 70% aggregate energy metabolism in the cerebrum of young rodents. Pertinent studies show that single-cell organisms like bacteria, insects and some rodents are proficient in acclimatizing and persisting of various types and times of food scarcity (14). During the sustained shortage, the different aspects of life can be presented to an extensive variety of situations radiation, warmth, low temperatures and different chemical exposures, to food shortage demands to undergo multiple stress-resistance approaches and reserve all the energy in defensive arrangements to limit harm, secure the hereditary components such as DNA and transferring across the generations should be unchanged (15). Experimental studies on yeast showed that when a single-celled being is transported from carbon or alcohol medium to H2O, it develops further resistant to the numerous stressful conditions and survives for a long time (16). Responding against food shortage, cells of yeast downregulate one or more primary ways, preliminary by Ras-PKA pathway to blood glucose response and secondary Tor-S6K pathway for amino acid response, and serine-threonine kinase pathway inhibition, the stress resistance transcription factors like Gis1 and Msn2/4, which are needed for the defensive mechanisms, to an extent by expanding the stress opposition qualities in genes including superoxide dismutase (SOD) and catalase (17). Outstandingly, when starving yeast cells reach a hypo-metabolic condition which enables yeast cells to limit the utilization of food sources reservation and is also able to gather abnormal amounts of the ketone body-like aceto-acetate, similarly for collection of ketone bodies in the mammals (18). Therefore, under stress conditions such as starvation or food deprivation, ATP consumption decreases (19). In a similar way, in respond against starvation, mammalian cells enter into a non-growing or a slowly-growing condition and put vitality assets into the cell defence facing different problems. Short amounts of insulin like growth factor (IGF-1) diminish mitogenic signalling pathways within the cells, down-regulating both of the main pathways downstream of IGF1R which are managed by Akt and Ras, as well as the ability to incite cell division cycle stimulated by p21 and p53 induction (20) and transcription of other defensive factors such as Egr1 and FOXO, to control SOD, and other stress resistance genes (21).
3. Cellular Modification and Related Metabolisms
Physical changes of micro-villi and epithelial tissues within each side of the gastrointestinal tract are observed inside the hens, maintained in fasting state for 12 h which is extended till 20 days, upon each fasting period. After the first 24 hours of starving, micro-villi displays a significant decrease inside the duodenum in terms of the villus size, slighter decrease in the jejunum. While there is not a significant decrease in the height of microvilli in the ileum. After 36 hours fasting, villi length in all small intestine parts gradually reduced during the next weeks, and notably, the villi stature of gastrointestinal tract enlarged upon the feeding by different forms of diet followed by 3, 10, and 12 days of abstaining food. Even after a long-term fasting (during 20 days), duodenum recovered rapidly, while a graduate recovery process is observed in the ileum, with a malfunction in absorption. Alternations of villus size inside the adjacent parts of the intestinal layer shows that the gastrointestinal absorptions are increased with the standard feeding state. All cellular contents as well as mitotic division, reduced significantly following the fasting status. Meanwhile, the decreased cell mitosis in proximal intestine recovers to the normal function after fasting, which shows that villi size is mostly changed in different epithelial cells (22). Interestingly, the epithelial cells of proximal intestine showed numerous large autophagolysosome vacuoles containing mitochondria as well as condensed organelles after 20 days of fasting. To note, size is decreased after the reefed of single time, showed fasting might lead to the breakdown of intracellular components via lysosomal enzymes by self-digestion (22). The ketogenic diet induces KBs formation within the hepatocytes from Acetoacetyl-CoA derived from beta-oxidation of free fatty acids (FFA) in mitochondria. A little amount of Acetyl-CoA enters into the tricarboxylic acid cycle, therefore acetyl-CoA would be more useful to form acetoacetate, or could be converted into BHB (beta-hydroxybutyrate) through BHB dehydrogenizing enzyme, otherwise converts directly into the acetone (23). Overall, KBs are distributed through the circulatory system in the brain and use as a potent energy source (24). Recent studies showed that astrocytes are able to intake KBs with the assistance of leucine and fatty acids (25, 26). Astrocytes serve a similar inclination against unsaturated lipids (instead of glucose) as a source of energy and enzymatic mechanisms in the cultured hepatocytes (27). Beta-hydroxybutyrate and acetoacetate enters into the cells through Mono Carboxylate Transport Protein (MCT), which gives an additional substrate to the brain. In addition, multiple studies described that KBs are a favourable source of carbon during glucose deprived situations (28), however, the complete underlying mechanisms of action remain still unclear. In this line, several findings declared that KBs is an effective energy source in comparison with glucose for all tissues, especially the brain. KBs are broken down and reabsorbed efficiently in comparison with glucose. KBs deviates the glycolysis and directly enters into the TCA cycle, while glucose initially enters into the glycolysis and TCA cycle, respectively (29). The KBs declined the glycolytic ATP while increases the ATP formation via oxidation in mitochondria (30, 31). In this regard, previous studies showed that equally glucose and KB are able to induce double molecules of Acetyl-CoA, in fact, four NAD+ are reduced by glucose and KBs (29). Ketogenic Diet (KD) is related to the regulation of hippocampal genes expression for mitochondrial and metabolic enzymes. KD additionally brings fatty acids which facilitate the induction of peroxisome proliferators-initiated receptor α (PPARα) as well as represse glycolysis and fatty acid synthesis, inducing transcription for catalysis of ketogenesis, causing fatty acid oxidation in mitochondria and peroxisome (32). In addition, cell viability is expanded accordingly from an increased phosphocreatine/creatine proportion within the hippocampal cells. According to the recent data, an enhanced fighting and acquired abilities against stressful conditions generated by metabolism malfunctions were observed in neurons which can act efficiently with an additional energy-efficient basis of fuel and with a greater load on mitochondria capacity, depending on the initiation of mitochondrial biogenesis (33).
4. The Generation of KBs
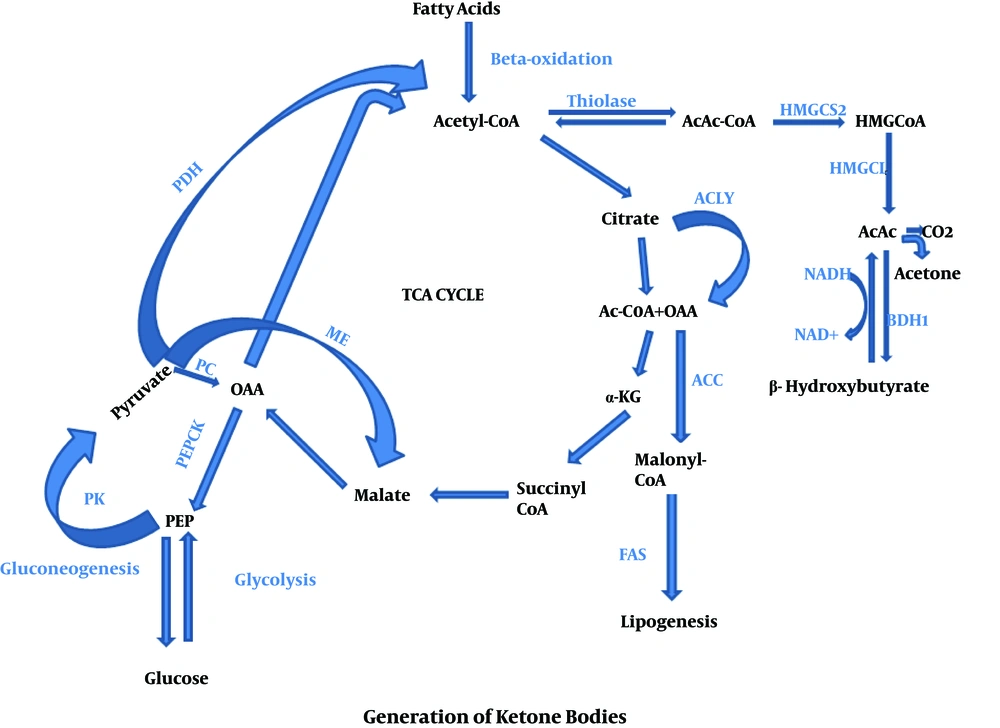
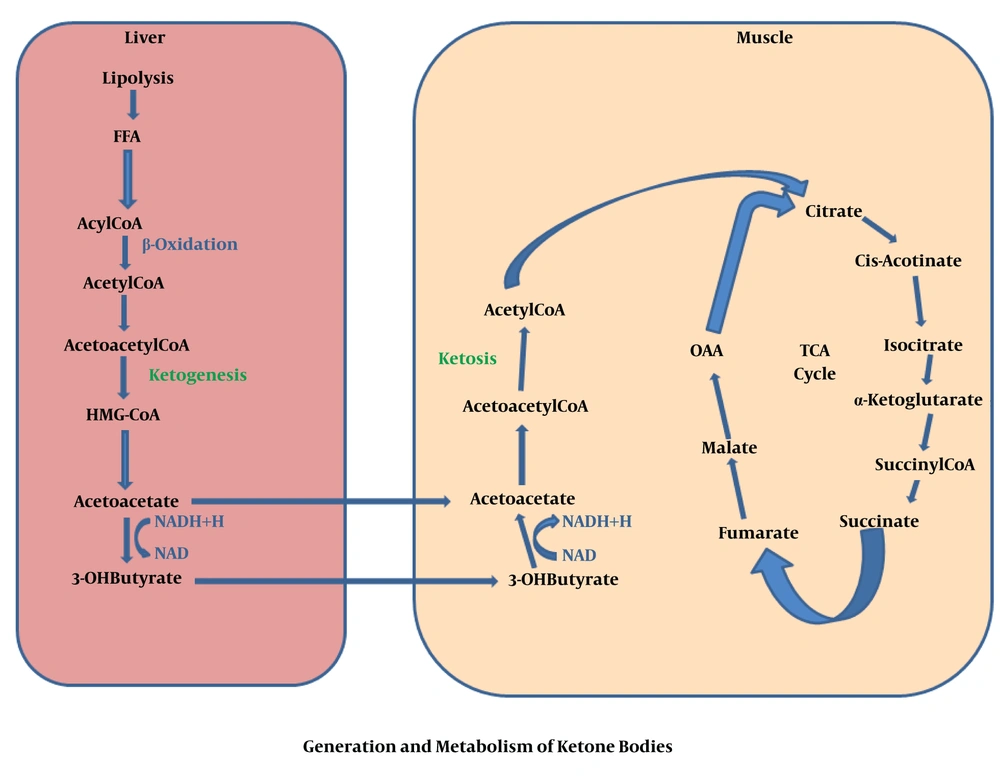
Generally, KBs are generated when glucose level decreases in blood, while fatty acid formation occurs via triacylglycerides break down inside the adipose tissues under the starvation condition. KBs are preferred by many tissues, specifically heart and skeletal muscles, in which fatty acids prevent glycolysis, afterward acetyl-CoA is produced via beta-oxidation of FFAs inside the liver tissue. Acetyl-CoA is a remarkable molecule, which plays a key role during cellular metabolism. A very high contribution of ATP is given by hepatic beta-oxidation needed to promote to the gluconeogenesis. A large number of Acetyl-CoA is changed into the KBs inside the peri-venous hepatocyte related mitochondria. Moreover, KBs amplify a functional form of fat-based energy source, because they are able to enter into the BBB, supplying the brain as an efficient energy source (6). In fact, fats are metabolized and result in the production of Acetyl-CoA through the mitochondrial β-oxidation. Inside the mitochondria, hydroxymethyl glutaryl Co-enzyme A (HMG-CoA) synthase accumulates Acetyl-CoA and acetoacetyl-CoA to produce HMG-CoA and acetoacetate molecules are released by HMG-CoA lyase. In this context, the most common precursor is acetoacetate, which leads to the formation of further two KB, acetone and BHB. After that, the acetoacetate is broken down by beta-hydroxybutyrate dehydrogenase to BHB. In fact, BHB is the most commonly found blood circulating KBs which hastily degraded into the acetone rather than acetoacetate. Once it enters into the target tissue, BHB converted again into the acetoacetate via a similar enzyme. Following this stage, the KBs uptake deviates from the other pathways. In brief, a CoA is transferred from Succinyl-CoA to the acetoacetate to produce acetoacetyl-CoA. This reaction is generally catalysed in most of the tissues via succinic-CoA: 3-ketoacid coenzyme A transferase, named SCOT or OXCT1. This enzymatic mechanism passes the essentially irreversible process which is catalysed via 3-hydroxy-3-methylglutaryl-CoA synthase (HMGCS2) (34). Finally, acetoacetyl-CoA converted into two molecules of Acetyl-CoA and then directly enters into the tricarboxylic acid cycle for ATP production and oxidation (34) which precisely was described in Figures 1 and 2.
5. Regulation of Ketone Bodies
The regulation of KBs synthesis is both extrahepatic and intrahepatic. However, the main producers of ketone bodies are long chain fatty acids (LCFA) during the post-absorptive period, and in all physiologic conditions related to hyperketonaemia, including the initial days after birth, LCFA concentration showing a gradual rising in the plasma (35). To note, isolation of LCFA from the hepatocytes depends on its concentration and there is a direct relationship between the LCFA concentration and ketogenesis in both mice and human.
6. Extrahepatic Pathway
For complete development, the main aspect that determines the transfer of LCFA into the liver depends on production speed by fat storage in adipose tissues. The decomposition of FFAs begins via adipose tissue related enzymes activation such as adipose triglyceride lipase and hormone sensitive lipase (36). In this line, adrenaline, noradrenaline, glucagon, and thyroxine hormone propagate the enzymatic function, but insulin and some eicosanoids show the reverse effects (37). Extracted human adipose cells from neonate showed sensitivity against the lipid lysing of thyrotropin (38). This is increased in large amounts solely after the birth, which can be participated for regulation of lipid breakdown during the prenatal period. If an abrupt reduction occurs in the blood glucose levels due to starvation, a high-fat diet accompanied by a gradual lessening of insulin in plasma causes a sudden increase to shift to the lipolysis as well as efflux of non-esterified fatty acids by adipose tissues. On the other hand, carbohydrate diet supplies the increased levels of insulin and then makes the cessation of lipolysis. In addition, the prompt KB synthesis is able to retort and reciprocate according to the amount of glucose presenting within the blood circulation. Therefore, it seems necessary to form an alternative energy source than glucose for the brain. During early stages of the rat infant, the ratio of insulin/glucagon favouring lipolysis is decreased (39). KBs are responsible for the regulation of its synthesis by different feedback mechanisms in assistant with adipose tissue to diminish the rate of lipolysis. Recent evidences suggest that there are two mechanisms: 1. Lipolysis which is directly inhibited by KB through binding with G-protein coupled niacin receptor; 2. Stimulation of insulin secretion through an indirect effect (40). While the experimental results on rat pancreas showed that KB could able to escalate insulin levels up when the concentration is over 1 mmol/L (41).
7. Intrahepatic Pathway
KBs are primarily formed and released by liver tissues which secretes them to the blood circulation, mainly because of relative specific hepatocyte expression of destiny fixing ketogenic mitochondrial enzyme 3-hydroxy 3-methylglutaryl coenzyme A synthase 2 (HMGCS-2). Meanwhile, the intestinal mucosa of neonatal rats is able to synthesize KBs via enterocyte-derived HMGCS-2 (42). In other words, the expression of HMGCS-2 in the intestinal mucosa is suppressed, while it is regulated by the gut microbiome in neonatal rats (43). The rate at which intestinal ketogenesis proceeds, is lower than 10% in comparison with the liver of animals at the prenatal stage, but this method provides a bonus strategy for distributing KBs to all developing cells (44). Long Chain Fatty Acids (LCFA) are first hydrolysed by triacylglycerols inside the adipocytes and then transferred to the plasma adhered with albumin, and then passes through the plasma membrane such as FFAs and after that binds with some binding proteins in the cytosol. Inside the liver tissue, LCFA either re-esterified to produce triacylglycerols, subsequently secretes a low-density lipoprotein and phospholipids. Next, it enters into the mitochondria via carnitine palmitoyltransferase (CPT) and undergoes beta-oxidation. Finally, all synthesized Acetyl-CoAs convert into KBs related acetoacetate and BHB consecutively are developed under four enzymatic reactions in the mitochondria stated as Hdroxyl Methyl Glutryl-Coenzyme-A pathway (45) as well as generally enters into the TCA cycle, in which undergoes terminal oxidation or will pass through the mitochondria in the form of citrate, thereby, it can be utilized in sterol and fatty acid formation as a cytosolic substrate (46). Medium Chain Fatty Acids (MCFAs) could not convert into tri-acyl glycerol inside the liver of mammals and straight cross the inner membrane of mitochondria by passing through the CPT system. Within the mitochondrial matrix, the MCFA converts into the acyl Coenzyme-A by-products via the enzyme acyl-Coenzyme-A synthase, and then undergoes beta-oxidation. KBs pass through the hepatocytes assistant with SLC16 transporter (A part of MCT family), and subsequently distribute in the blood circulation received by the extrahepatic cells, and ultimately oxidized by MCT-related mechanisms (47). The processes of KBs transferring to mitochondria are remaining unestablished.
8. Extra Benefits Effect of Ketone Bodies
8.1. Anti-Seizure Effects
A specific diet which is successfully utilized to help patients with epilepsy comprising a low glucose index treatment (LGIT) which is not able to cause systemic ketosis (48). Recent studies also revealed that KBs specifically BHB exert anti-seizure effects (49). Moreover, scientific evidences for both KBs declared the possible neuroprotective as well as anti-seizure properties in copious amounts over the past few years (2). According to the previous studies, aceto-acetate and acetone but not beta-hydroxybutyrate showed protective effects against hearing disorders in seizure susceptible mouse model (50). Later, the acetone showed increasing threshold of seizure in multiple seizure inducing models of rats such as several tests containing maximum electroshock test model's grand mal seizure, pentylenetrazol testing for petit mal seizure, kindling test of the amygdala for focal impaired awareness seizure as well as AY-9944 test for atypical petit mal seizure (51).
9. Management of Neurological Disorders
Some reports support the reestablishment of motor connections with the assistance of KD in rat models of AD with spinal cord injury, Parkinson disease, and Rett syndrome (RTT). Furthermore, various models of mouse affected by AD caused by mutation of amyloid precursor peptide, presenilin taken as amyloid depositing model and a model for tau protein deposits (Tg4510) have shown increasing latency for dropping on rotarod instrument with ongoing KD without reducing amyloid-beta or tau (microtubule-associated protein in neurons) formation (52). KD also promotes the front limb motor overactivity in animal models after spinal cord injury and keeps the functional characteristics when returned to the standard diet 12 weeks after the exposure (53). In this respect, some studies reported that KD and beta-hydroxybutyrate intaking prevents dopamine synthesizing neurons from degeneration (54) with increase motor functions in rat models suffering from PD (55). Rehabilitation in motor function is also illustrated in mice with RTT, mainly by omitting calories from the normal diet (56).
10. Impacts on Diabetic Heart
Diabetic heart is characterized by insulin failure, increasing uptake of glucose and highly relies on fatty acids as a source of energy in animal models. To date, it is not completely known how cardiac metabolism changed under diabetic conditions. However, the assessment of diabetic cardiac energy metabolism comparing with non-diabetes is performed underwent cardiac catheterization for heart diseases. Glucose, lactate, and pyruvate uptake by myocardia is reduced while KBs such as beta-hydroxybutyrate and aceto-acetate are heightened in patients with diabetes in comparison with a non-diabetic individual. However, the myocardial uptake of FFAs is not considerably increased in diabetic in comparison with non-diabetic cardiac tissue (57).
11. Ketone Bodies Decreasing Tumor Cells Proliferation
A specific metabolic activity is expressed by cancer cells. To better words, a robust increase in glucose consumption happens due to the mutation in genes resulting in mitochondria malfunction. Studies showed that cancerous cells cannot use KBs for energy generation; therefore, slowing down or stopping the division of cancer cells. Based on in vitro studies, KBs showed a declining trend in the cultured tumor cells viability. It is proposed that the Warburg effect is a known metastatic character of cancer cells. In vivo studies also showed that KBs is able to block the metastatic cell growth. Rapid cell growth is observed in the metastatic Vm-M-3 cell line grown in the presence and absence of beta-hydroxybutyrate. In detailed, a mature male mouse was implanted with luciferase tagged Vm-M-3 cells and fed with diet consisting 1, 3-butanediol, and ketone, which were broken down into BHB and aceto-acetate.
12. Ketone Bodies Related Side Effects
There is a little published data regarding the KBs side effects, which mostly depend on prolonged administration through the gastrointestinal tract than the subcutaneous (SC), intramuscular (IM) routes. under severe condition, H+ concentration, Na+ level, fatty acids and carbohydrate are affected by the parenteral formulations which depend upon the concentration of KBs. For instant, the rate of alkalinisation in intravenous (IV) formulations increase. This study conducted by Hiraide et al., reported the robust increase of H+ and Na+ concentration followed by IV uptake of sodium BHB solution (20%) in severe trauma patients (58). To note, descending rate in glucose brain metabolism and the escalation in brain blood circulation is a potential concern confirmed by Hasselbalch et al., in IV administration of BHB (59). However, the long-term consequences induced by KBS are still unknown. Although a few adverse effects are expected, followed by the administration of KD gastrointestinal tract such as hypoglycaemia and dehydration. Moreover, some adverse effects could be emerged, including obesity, bone density reduction, growth retardation, nutrient deficiency, hepatic failure, and immune dysfunction upon the chronic use. There is a remarkable increase in average cholesterol concentration over 250 mg/dL observed in blood circulation followed by the long-term consumption of KD (60). It has been proposed that KD can be atherogenic leading the deposition of lipid and atherosclerotic plaques in the blood vessels. In patients with KD has been shown the report with dilated cardio myopathy caused due to the high level of FFAs into the plasma. Moreover, the elevation of uric acid in serum and nephrolithiasis has been reported in patients under KD (61).
13. Transcriptional Regulation of Ketogenesis and Ketolysis
Despite calorie restrictions (CR), 1029 genes of adipocytes, as well as 1831 genes in hepatocytes have different gene expression patterns. In this regards, gene expression is not significantly affected by MC4R genotype. Calling attention, starving stimulates various gene expressions suggesting the steroid and lipid formation, which is down-regulated equally in hepatocytes and adipose tissues. The food regimen augmented the gene expression participating in the pathways of glucose sparing, for example, oxidation of amino acids and FFAs in liver and pathways involved in the extra-cellular matrix (ECM) such as adherent connection between adipocytes and cell adhesion. It is also identified that different gene expression patterns of transcription factors are responsible for regulating numerous expression genes. It ensures that transcription factors including SREBF1, PPARG and CEBPA, are recognized for regulating the fasting response and implicate other factors of transcription like ESR1. Fascinatingly, ESR1 manipulates some starving stimulated genes in fats, participating in the morphogenesis of cytosol (62).
Furthermore, endocrine secretions and growth factors are responsible for providing appropriate enzymatic machinery for KB synthesis and its breakdown by reprogramming of genes for transcription. In the meantime, the most important transcription factor is PPARα which induces the promotion of most genes required to transfer FFAs, their uptake and oxidation with the biosynthesis of KBs (63). PPARα is known as nuclear receptor related to endogenous ligands are made up of FAs and their products (64). PPARα regulation with the main transcriptional genes occurs when retinoic X receptor (RXR) and heterodimeric complex of ligand-bound PPARα bind with peroxisome proliferators responding elements into the gene inducers. Interestingly, different co-activator proteins are recruited by the complex of PPARα-RX with a histone acetyl-transferase action which belongs to the CBP/p-300 or SRC/p-160 family (65). These events enable a common transcriptional complex formation associated with promoting and chromatin remodelling. The importance of PPARα in coordination with cellular response against fasting was emphasized in knockout rats. These animals abruptly progressed hypoglycaemic condition following glucose deficiency as well as ketogenesis failure regarding the hepatic fatty acid oxidation under the starvation condition (66). Among different PPARα endogenous agonists, derivatives of LCFA such as acyl-ethanolamines or Acyl-CoA esters released via mobilization and lipid degradations, are observed as potent stimulators. Once PPARα binds with ligand tightly results in activation of genes expression including carnitine palmitoyl transferase 1A (CPT1A), fatty acid-binding protein (FABP), mitochondrial lengthy, medium-chain Acyl-CoA dehydrogenases (LCAD, MCAD), peroxisomal acyl-CoA oxidase and several rate-limiting enzymes for ketone synthesis such as hydroxymethylglutaryl-CoA synthase (HMGCS2). In addition, PPARα plays an important role in initiating the transcriptional regime for ketosis, while HMGCS2 considered as milestone in the ketogenic pathway, is highly controlled by various post-translational mechanisms as well as transcription factors. Consensus peroxisome proliferators responsive element (PPRE) is present in the Hmgcs2 gene and its sequence is localized at 104-92 base pairs in the promoter region (67). HMGCS2 protein is able to bind with PPARα and complexis transported inside the nucleus through PPRE and related trans-activates the Hmgcs2 gene. This is the most fascinating mechanism of Hmgcs2 transcription (68). HMGCS2 protein consists of a canonical motif which is responsible for interacting with the nuclear receptors, LXXLL, as nuclear receptor interaction motif (69), which has potential to form a complex with PPARα. Interestingly, this motif is not essential for interacting but palmitoylating certain key cysteine residues 305 and 166 in animal protein. Solitary palmitoylated HMGCS2 protein can only bind with PPARα and trans-activate itself successfully (70). Except PPARα, other transcription factors are also able to affect Hmgcs2 transcription either negatively or positively. Positive regulators comprise SP1, CREB, COUP-TF and forkhead family-related transcription factors (FOXA2) and DKHRL1 (71). To note, the antagonist of hepatocyte nuclear factor 4 (HNF4) is able to suppress Hmgcs2 transcription (72). The regulation of ketogenesis is performed via observing the total consumption of KBs by extrahepatic cells. During fasting, MCT1expression reached high levels. It is the main membrane transporter that performs KB absorption, which leads to the efficient import of KBs and ketone degradation. Notably, MCT1 is a widely expressed gene that consists of PPRE as a promoter and easily trans-activated either by PPARα ligands or food deprivation (63).
14. Conclusion
KBs are remarkable molecules which show many neuro-protective effects. It has been documented that KBs have a strong potential impact on improving neurological disorders. It can also be used by an individual for a short period of time on purpose, in a highly regulated manner either it is for fat reduction or improving efficacy in the brain because KBs lead to much more production of ATP than a molecule of glucose. KD allows us to generate KBs in a controlled manner. In several studies, it has been observed that KBs suppressed the proliferation of cancerous cells. Moreover, KD could be used as additional therapeutic agents along with chemotherapy for an effective treatment of cancers beside the detection of tumor cell biomarkers. Interestingly, several enzymes are produced by a cancer cell to metabolize KBs for energy production, but still, they are not as efficient as a normal cell. After characterizing the cancer cells, a specific drug can be given which will block the enzymatic activity (ketone metabolizing enzyme). It should be noted that cancer cells will be destroyed by either food deprivation or extra accumulation of KBs, which leads to an acidic condition known as ketoacidosis. Besides all advantages, there are some adverse effects that should be addressed. It also requires more studies and attention for further determination of potential advantages of this molecule which cannot be ignored.