1. Background
With the rapid growth of communities, greater amounts of pollutants are released into soil or water areas, which do great harm to human health as well as ecosystem (1, 2). In the past decades, the release of dye effluents from industries such as textile, paper and pulp, leather, food, cosmetic, and etc., has become one of the major environmental concerns owing to their carcinogenic, genotoxic, and/or mutagenic nature (3, 4). About 20% to 50% of used reactive dyes in textile mills can be released in aquatic environments (5). Reactive dyes are usually soluble in water and they applied for dyeing of cellulose fibers such as rayon and cotton; however, they are also used for leather, nylon, silk, and wool (6). There are several methods available for removal of dyes from wastewater such as adsorption with activated carbon, biological oxidation, and chemical coagulation (7, 8). Biological methods have limitations in applying for textile effluents because of toxic effects of commercial dyes for microorganisms (9). Chlorine, as an oxidizing agent in chemical degradation, is one of the most effective and important methods, however, it has some disadvantages like production of some toxic outputs (10). Adsorption of pollutants by activated carbon associates with high production cost (9), difficulty in the process of regeneration, and waste disposal high costs. Electrocoagulation (EC) is one of the best and efficient processes in dye removal and other organic pollutions from industrial effluents (10). EC used for the treatment of various types of wastewater such as textile (11, 12) dairy and tannery wastewater (13), laundry wastewater (14) and pulp and paper mill industry wastewater (15). The present method is specified with simplicity in apparatus, ease of operation, decreasing or lack of equipment for chemical injection, and reduction in sludge amount that is quickly settled (16). EC uses a direct electrical current source between metal electrodes that are partially immersed in the electrolyte. Electric current causes the metal plates to be dissolved in the wastewater (17, 18). Two widely used metals in electrocoagulation process are iron and aluminum (11). The main processes that happen during electrocoagulation, are: (a) electrolytic reactions on the electrode surface, (b) formation of coagulants in the liquid phase, and (c) adsorption and removal of colloidal or soluble pollutants by flotation or settling (9). Various reactions occur in the EC process, where iron is used as the electrode.
Anode:
Cathode:
Overall:
In recent years, electrochemical methods and advanced oxidation processes (AOPs) have been developed for treatment of drinking water pollutants and industrial wastes (19, 20). AOPs, based on sulfate radical, are usually used for destroying organic pollutants. Sulfate radical (SO4•−) is a powerful oxidizing factor, which has a redox potential (E0 = 2.5 - 3.1 V), which is comparable with hydroxyl radical (E0 = 1.8 - 2.7 V). Activation of persulfate can be done in various ways, such as UV irradiation, transitional metals, heat and electrochemistry, and then lead to sulfate radical generation. UV irradiation for sulfate radical production is an appropriate environmental method (15, 21).
The removal equation as indicated in Equations 8
2. Objectives
Due to the advantages of EC process and low chemical consumption in this process, this method was first used to remove reactive blue 52 and then, the effluent from it poured into the UV/persulfate process to further improve the dye and COD removal efficiency. Parameters investigated included the pH, current density, initial dye concentration in the EC processes, and pH and persulfate concentration in the UV/persulfate processes.
3. Methods
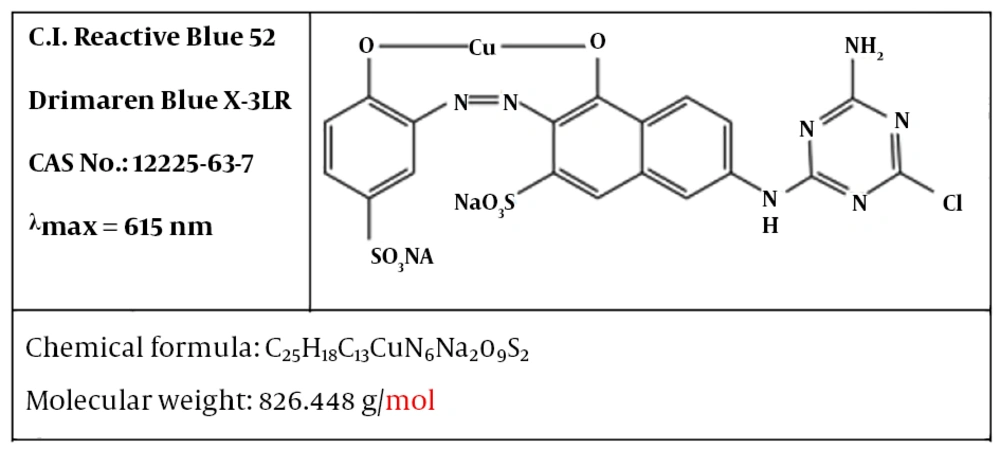
The reactive blue 52 dyes (RB 52) were purchased from the Alvan Sabet Company. Figure 1 displays the molecular structure of this dye. Sulfuric acid (98%), sodium hydroxide, and sodium persulfate was purchased from the Merck Company. For preparation of dye solution, solid dye was dissolved in distilled water. In order to adjust conductivity of solution, 0.3 g of NaCl was dissolved in synthetic dye solutions (volume 1 L).
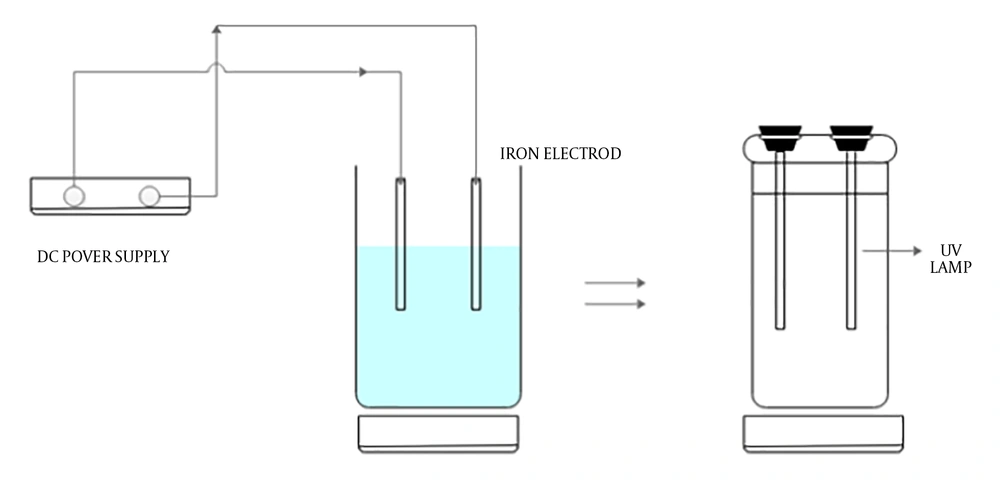
The experimental setup is shown in Figure 2. The EC tests were performed in a cylindrical glass reactor (dimensions: 14.5 cm height × 10.5 cm radius). The iron electrodes with the same dimensions (2.5 cm × 16 cm × 1 mm) were used both as cathode and anode. The total effective area of electrode was 60 cm2 and between the electrodes was 20 mm space. Adjusting of solution pH was done by NaOH (1.0 M) and sulfuric acid (1.0 M). For reduction of mass transport over the potential of the EC reactor, the solution was agitated continuously at a rate of 200 rpm by a magnetic stirrer (model: Heidolp 3600). The total volume of the EC reactor was 1250 mL, where its net volume for reaction was 1000 mL. By using a DC power source (model: DAHENG PS 302D) the cathode and anode were connected to the negative and positive outlets. At the end of each run, the resulting effluent was centrifuged at 3000 rpm for four minutes. A total of 600 mL of the effluent resulted from the previous stage (EC) was poured into the cylindrical photo reactor. Two UV-C lamps (6 W) was placed in a quartz tube in the middle of the reactor as a UV source for activation of sodium persulfate. After adjusting the pH, certain amounts of sodium persulfate were added to the resulted solution. Then, by turning on the UV-C lamps the reaction was started. During the process and when the effluent was fertilized, at different intervals, samples were collected.
For determination of dye concentration in the initial and treated effluent, a UV-vis spectrophotometer (DR 5000) was used, which calibrated at 615 nm (as maximum absorbance wavelength). For calculation of dye removal efficiency (RE%) Equation 10 was used.
Where C0 is the initial dye concentration before reacting, and Cf is the final dye concentration after reaction.
4. Results and Discussions
4.1. Electrocoagulation
4.1.1. Effect of Initial pH on Dye Removal Efficiency
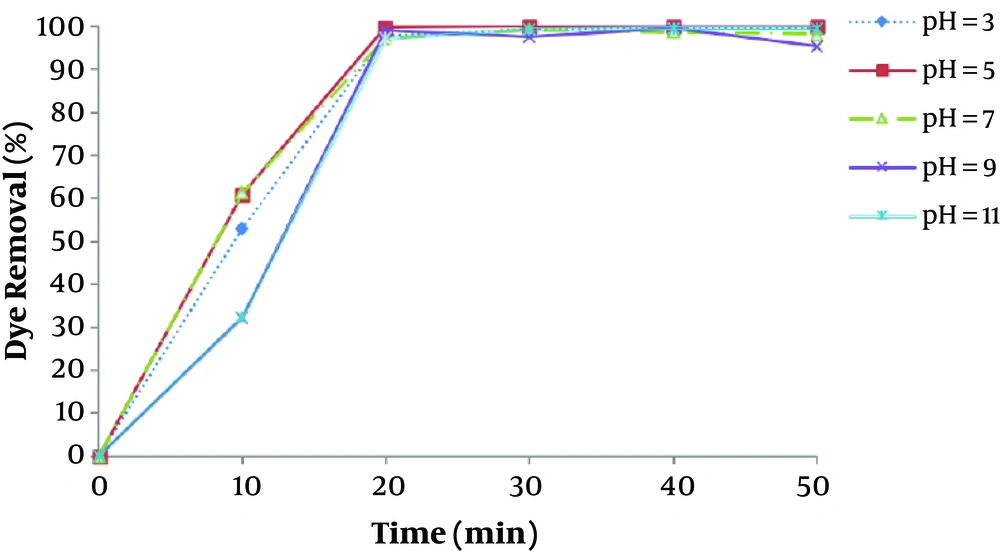
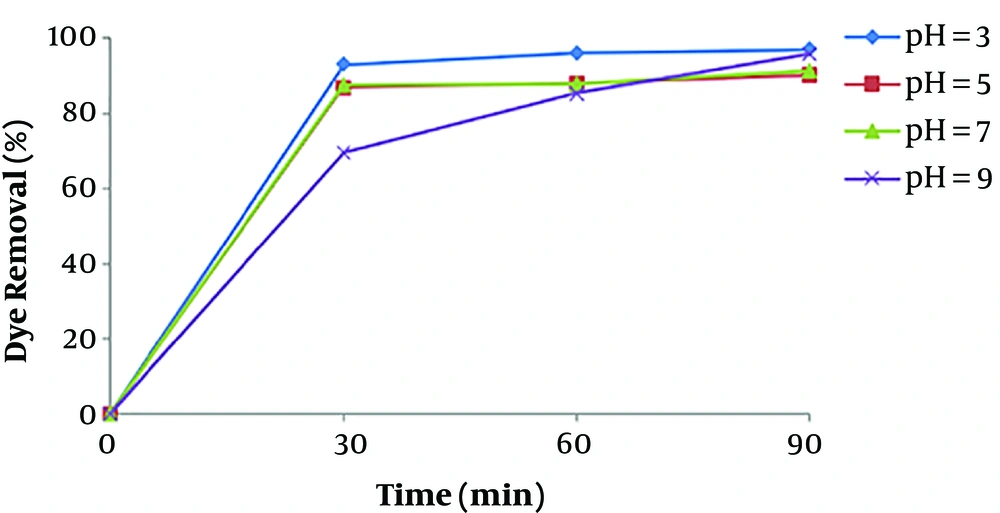
pH is one of the most important factors that affect the performance of chemical processes such as EC, as has been observed in other similar studies (12, 22). The removal efficiency as a function of pH was shown in Figure 3. The current density was maintained at 16.66 mA/cm2 for the study of pH effect. Figure 3 illustrates that the maximum removal efficiency of dye is observed at the pH of about 7, which is a nearly neutral range. This can be explained in this way that the degree of solubility Fe (OH)3, beyond the pH value 7 increases as a result of soluble Fe (OH)4 formation, which doesn’t participate in the COD reduction (23). At acidic conditions, a large amount of irons in the form of Fe2+, that is soluble and stable in the environment, causes reduced formation of coagulant and thus, dye removal (24). At pH values greater than 10, Fe (OH)4 species are dominant, which aren’t appropriate coagulant agents.
The results of a study by Daneshvar et al., revealed that maximum basic dye removal efficiency by electrocoagulation was occurred when dye solution pH was in the range of 5.5 - 8.5.
4.1.2. Effect of Current Density on the Color Removal Efficiency
Almost in all EC processes, density of the current is one of the most important parameters to reaction rate control the reactor. This behavior is due to the intensity of the current that determines the dosage rate of coagulant, rate production of the bubble, and growth of floc size resulting in faster removal of pollutions (5). The release rate of iron ions into the solution from the iron anode follows Faraday’s law that is expressed as:
Where C is the iron concentration in the electrolytic cell, I the current, t the retention time, M the molecular weight of anode (iron), Z the chemical equivalence, F the Faraday’s constant, and W the volume of the electrolytic cell.
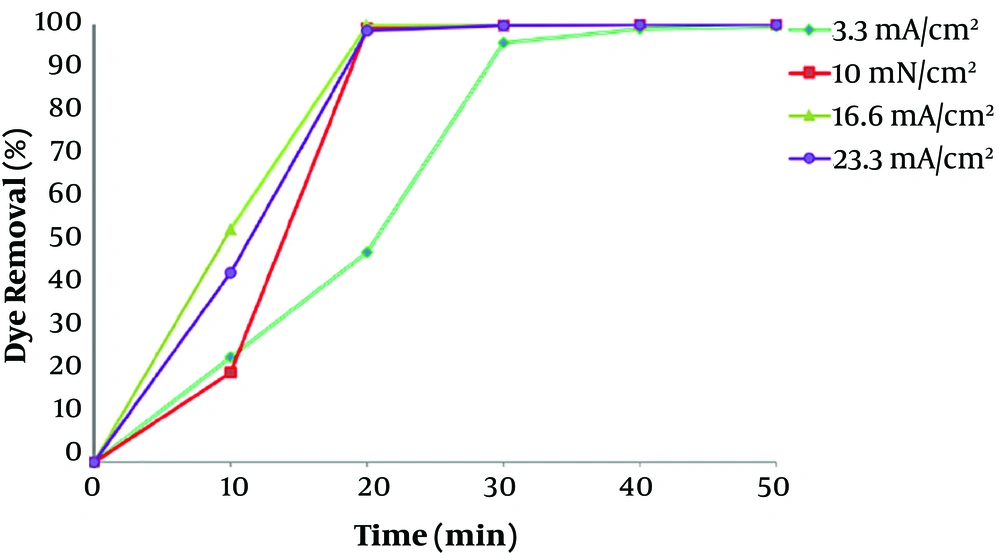
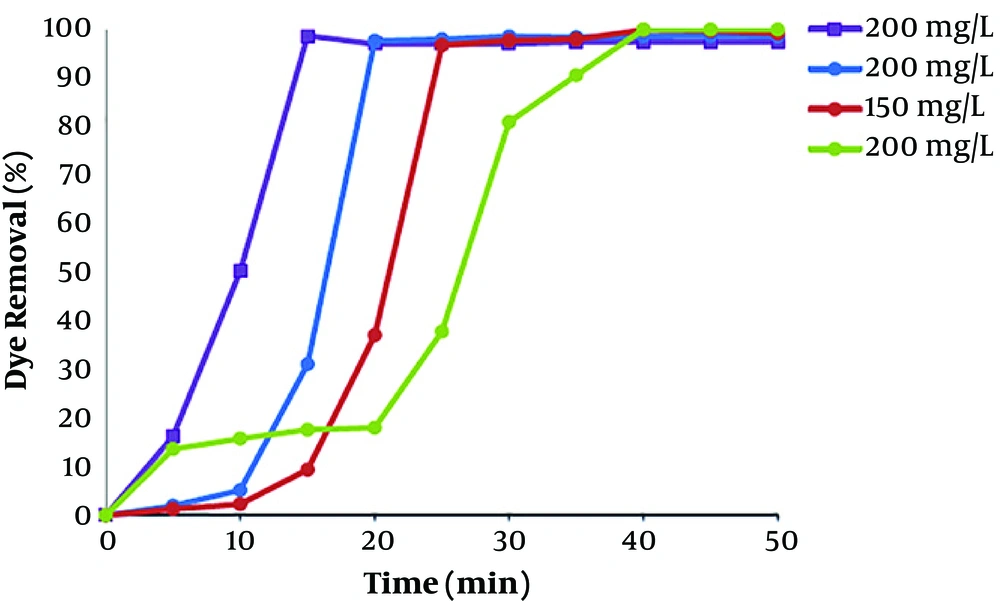
To investigate the influence of current density for the removal of dye, electrocoagulation approach was done in different currents at constant initial concentration of 100 mg/L at pH 7. Difference in dye removal efficiency, with contact time at various current values in range of 3.33 - 23.33 mA/cm2 (I = 0.1 - 7.0 A), is provided in Figure 4. It shows that the rate of dye removal increases with enhancement in current values. Due to high current concentrations, the amount of anodic iron dissolution increases, leading to a higher amount of sediment for the removal of dye (25). Kartikaningsih et al., showed in their study that when the current density is increased from 1.25 to 5 mA/cm2, the removal efficiency from 72% to 95% increases (26). Therefore, the adsorption of dye molecules also increases with increasing the Fe hydroxyl. As the results showed, electrocoagulation process has lower efficiency at lower current densities, due to the less iron releasing from the anode, and therefore, the removal of color was low. However, after 30 min of the process, the removal efficiency of dye was more than 99 % for all current densities above 10 mA/cm2. Therefore, the optimum current density of 10 mA/cm2 was carried out for the dye removal from polluted solutions including RB 52.
4.1.3. Effect of Initial Dye Concentration
The dye solution, with various initial concentrations in the range of 50 - 200 mg/L was degraded by EC in optimum pH range and current density of electrolysis values. As the results showed in Figure 5, efficiency of dye removal reduced with increasing initial dye concentration. This phenomenon is due to the reason that at a constant current density and time, the amount of iron hydroxide producing is the same in all dye concentration. As a result, the produced flocs has the same amount in the solutions. Consequently, the produced flocs at higher concentration of dye were inadequate to agglomerate most of the dye molecules in the solution. The result of the study of de Carvalho et al., shows decreasing in dye removal efficiency due to the increase of the dye concentration in solution. The higher number of iron hydroxide, rather than dye molecules in lower dye concentration, causes 100% dye in a short time compared to higher concentration. Kobya et al. (23), and Nandi and Patel (27) revealed similar results in the degradation of reactive dye and brilliant green dye from aqueous solutions by EC process. Kobya et al., showed in their study that decolorization efficiency by Al electrode in electrocoagulation process from 99.6% to 88% with an increase in dye concentration from 100 to 500 mg/L (22). Nandi and Patel found similar results after 30 min of process, degradation of dye decreased from 99.87% to 67.78% if the initial concentration of dye was increased from 50 to 200 mg/L (27).
4.2. Advanced Oxidation (UV/Na2S2O8)
4.2.1. Effect of Initial pH
The electro-coagulated effluent was introduced to the photo reactor for evaluation of UV/PS effluent from the electrocoagulation process was returned to the photo reactor for assessment of the UV/PS process. The influence of pH and dose of PS was examined. Figure 6 shows the influence of pH on removal of dye from electro-coagulated effluent. The dye removal efficiency decreased with increasing pH from 3.0 to 9.0 and has been revealed in other studies, sodium persulfate shows the optimum efficiency at neutral condition (28, 29). A similar finding was reported by Lin et al., who found that the degradation efficiencies of PVA after 30 min at pH 3, 7, and 11 were 94%, 53%, and 23%, respectively; obviously, the degradation efficiency of PVA after 30 min increased as pH declined (30). In AOPs, the value of pH has a direct effect on the production of hydroxyl and sulfate radicals in solution. This trend may be due to further production of sulfate radicals in acidic conditions, which are inclined to neutral conditions. Therefore, sulfate radical production is completely dependent on pH in these processes. At a pH below 7 (particularly from 3 to 5) sulfate radical is dominant (reaction 12 and 13) under this range of pH conditions, the degradation of persulfate into sulfate free radicals can produce more SO•4 (31). At a pH between 7 to 9 both radicals (sulfate and hydroxyl) are existed that have the potential to generate a rapid attack on RB52 molecules (reaction 14). However, in exhaustive alkaline conditions OH• is dominant. In high pH, sulfate and hydroxyl radicals had a scavenging reaction together (reaction 13), which as a result, decreases the efficiency. The presence of SO42- can cause limitation of the reactivity of OH• or SO•- complexes (29). This reaction significantly reduces process efficiency because the oxidation reactions were being carried out in the absence of radicals (32).
4.2.2. Effect of Sodium Persulfate Concentration
In the process of UV/Na2S2O8, PS dose has an important role in creating the free radicals and removal dye. The influence of sodium persulfate (PS) concentration on the removal efficiency of dye in UV/Na2S2O8 process is illustrated in Table 1. Raising persulfate concentration from 5 to 15 mmol/L increased removal efficiency from 54.71% to 98.55% after 30 min. The formation rate of SO•4 increased by increasing the PS concentration. SO•4 is considered as a strong one-electron oxidant that can react to the color to produce the color radical cation (reaction 16).
| Time (min) | pH | PS (mmol/L) | Dye Removal (%) |
|---|---|---|---|
| 30 | 7 | 5 | 54.7 |
| 60 | 7 | 5 | 60.63 |
| 90 | 7 | 5 | 67 |
| 30 | 7 | 10 | 86 |
| 60 | 7 | 10 | 88 |
| 90 | 7 | 10 | 91.4 |
| 30 | 7 | 15 | 98.55 |
| 60 | 7 | 15 | 100 |
| 90 | 7 | 15 | 100 |
| 30 | 7 | 20 | 97.2 |
| 60 | 7 | 20 | 98.4 |
| 90 | 7 | 20 | 99.2 |
Though, with increasing PS dose to 20 mmol/L, the trend of dye removal decreased compared to the PS dose of 15 mmol/L in this process; by increasing concentrations of persulfate from 15 to 20mmol/L, the removal efficiency reduced from 98.55% to 97.2%. The best concentration of S2O8− can therefore, be 15 mmol/L, since a removal dye efficiency (98.55%) can be attained with this value after 30 min and additional amounts do not much progress this trend. This results in optimum condition is close to the results of study by Jaafarzadeh et al. (15), which combined 15 mmol/l of persulfate ion as the ideal amount in joined electrocoagulation and UV-based, sulfate oxidation methods for treatment of pulp and paper wastewater. More PS can scavenge sulfate radicals and S2O8•− is created based on (reaction 17) that is a weaker oxidant compared to sulfate radical (15). Also, this behavior observed in the Astereki et al. study to remove 2-chlorophenol of aqueous solution using the advanced oxidation processes resulting from iron/persulfate and UV/persulfate (32).
5. Conclusion
The combination of EC and advanced oxidation (UV/persulfate) has been applied to removal reactive blue 52 from aqueous solutions. The optimal condition was obtained at neutral pH, 10 mA/cm2 and 30 min reaction time in electrocoagulation process. Under this condition, 82/4% RB 52 removal efficiency was achieved by EC process. The attained wastewater effluent from the EC was treated by sulfate radical-based UV/Na2S2O8 processes. UV/Na2S2O8 had the best performance in natural pH (pH = 7 and Na2S2O8 = 15 mM/L), while scavenging effect was observed in excessive persulfate concentration. In combination of electro-coagulation and UV/PS processes the removal efficiency of 98%/86% was attained. Such combined process of electrocoagulation and advanced oxidation proved to be efficient and presents potential applications in industrial scale.