1. Background
Over the past three decades, the rate of environmental pollution has escalated dramatically. In this context, noise pollution is considered a widespread and global problem in most countries (1-3). Noise pollution affects social well-being and is an important criterion for determining the quality of life (QoL) in many cities (4-8). Noise is considered as one of the stressors in the industry (8) and is the second most important cause of heart attack after smoking and air pollution (9). Sound pollution is considered the most dangerous pollutant in big cities (10). Moreover, noise has indirect effects on human performance, and it can increase the risk of accidents and errors due to reduced focus (11-13).
Noise annoyance as a measurable mental response has serious adverse effects and could trigger other adverse effects as well (14, 15). The human body’s response to noise is the same as a response to an imminent danger. These reactions include hormone secretion, heart rate, and blood pressure alterations (16). One of the effects of exposure to noise is hormonal imbalance. Sound causes changes in the secretion of various hormones in the body, and this, in turn, may trigger other direct and indirect effects.
In addition, hormonal imbalance is affected by factors such as lifestyle, type of nutrition, pollution, mental states, and age of an individual. One of the most important signs of hormonal irregularity is sudden anxiety or depression. Depression is the most common psychiatric disorder and has recently been associated with hormonal imbalances (17). Today, depression is a serious and widespread problem (18). The World Health Organization’s report in 1997 indicates that depression is the fifth-highest outbreak among other diseases and will be ranked second by 2020 (19). Many studies have indicated the effects of thyroid hormones on mood and behavior so that 5 - 10% of people who are being assessed for depression reveal thyroid dysfunction (20). The thyroid function test is one of the most common laboratory tests usually done to investigate mood disorders. There is evidence that hypothyroidism can cause signs and symptoms of depression in humans (21). Some studies have shown increased T4 levels during the depression, while others have not shown this correlation (22). Several surveys have shown different findings, such as increased T4 and TSH; however, other studies have indicated lower levels of T3 and TSH (23, 24). In addition, some research projects reported high levels of total thyroxin and free thyroxin in acute depression (22). In general, hypothyroidism is associated more with depression, and hyperthyroidism reduces sleep and causes restlessness and irritability (25).
2. Objectives
3. Methods
3.1. Experimental Design and Housing Conditions
Male Wistar pathogen-free rats weighing 200 - 250 g were purchased from Razi Institute, Mashhad, Iran. They were housed in polypropylene cages (400 × 250 × 150 mm) with steam-cleaned pinewood bedding at 20 - 22°C with 40 - 50% relative humidity (10 times/h air displacement) in a controlled animal house. They were in a 12: 12 hour light/dark cycle (from 8.00 p.m. to 8.00 a.m.) during exposure, and food (rodent chow; Pars Animal Co., Iran) and tap water were available ad libitum, except during exposure.
All experimental procedures were approved by the Research Ethics Committee of Gonabad University of Medical Sciences, Iran. The Declaration of Helsinki guidelines was respected throughout the study.
3.2. Procedures
In this experimental study, 70 male rats were randomly assigned into seven groups (one control and six experimental), so that the control group was not exposed to any type of sound wave. The grouping of rats is shown in (Table 1).
| Group Number | Noise Level (dB) | Exposure Time (Minutes) |
|---|---|---|
| 1 | 60 | 30 |
| 2 | 60 | 60 |
| 3 | 85 | 30 |
| 4 | 85 | 60 |
| 5 | 110 | 30 |
| 6 | 110 | 60 |
| 7 a | 0 | 0 |
a Control group.
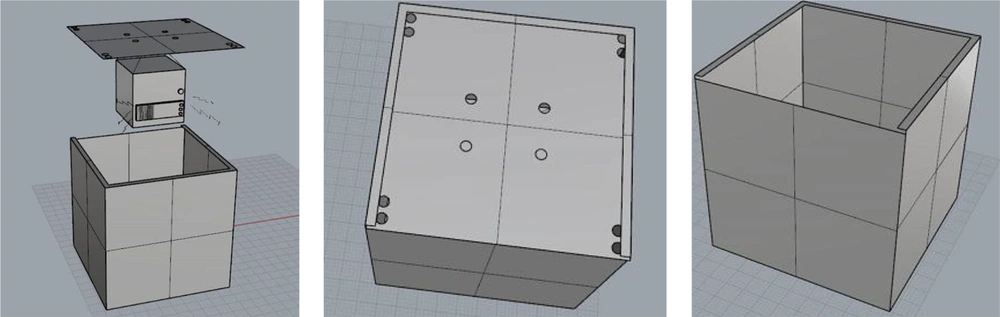
All experimental and control groups were stored in acoustic chambers designed for this purpose, and their environmental conditions were controlled. Exposure to sound was carried out in acoustic enclosures. The chamber was made of wood that had ventilation holes on the ceiling for the airflow, and small light bulbs were installed inside the chamber. Due to the deletion of reflective sound and the change in frequency and intensity of the sound, its inner surface was covered with sound-absorbing material.
The white noise that is a random signal having equal intensity at different frequencies was generated by Noise.exe software (28), then recorded and played by a sound player mounted on the ceiling of the chamber. To determine the accuracy of the sound intensity and frequency, sound measurements were performed according to ISO7216 standards using the CELL_450 made in the UK. The Casella CEL-110/2 Sound Level Calibrator was used to calibrate the sound level meter device according to the manufacturer’s instructions. The sound player was installed so that the sound was uniform throughout the chamber compartment. The schematic view of the designed exposure chamber is shown below (Figure 1).
All groups were kept in this chamber for half an hour before exposure to noise. Then, the six experimental groups were exposed to noise at different levels and exposure times. All groups were exposed to the white noise for 50 minutes per day. Sound level and exposure time were specified for each group according to the schedule. All groups were exposed at a specified hour to the defined sound. Blood samples were collected after anesthesia using Ketamine (100 mg/kg), and Xylazine (10 mg/kg), and the serum samples were stored at -70°C until analysis. Serum levels of hormones including thyroid hormones (T3 and T4) (Pishtazteb, Iran), TSH (Cusabio, China), cortisol (IBL international GMBH, Germany), and testosterone (IBL international GMBH, Germany) were determined based on the ELISA method. Except for the rat TSH (a polypeptide hormone), which is different from the human TSH, other hormones are the same. In the current study, to determine the concentration of hormones other than TSH, human assay kits were used. The principle for TSH assay was sandwich immunoassay, so that a TSH-specific antibody was coated at the bottom of each well. After the addition of serum-containing TSH molecules, the second TSH-specific enzyme-conjugated antibody was added. After incubation, unattached agents were washed, and then the substrate was added. After a few minutes, the enzymatic reaction was stopped using the stop solution. The developed color was measured using an Anthos 2020 microplate reader (Biochrom, UK). The principle for the other thyroidal and steroidal hormone assays was competitive immunoassay. In this method, a given-hormone specific antibody was coated at the bottom of each well. After the addition of serum-containing hormone molecules, the conjugate reagent containing the HRP-conjugated hormone was added. After incubation and washing unattached components, other steps were performed similar to the TSH assay mentioned earlier. To determine the concentration of the hormone in each sample, the color intensity for each sample was compared with the standard curve.
3.3. Statistical Analysis
All statistical tests were performed in SPSS software version 21. The normality of data was determined using the Kolmogorov–Smirnov test. Descriptive statistic tests were used to observe the mean and standard deviation of the quantitative variables. To determine the relationship between quantitative normal variables and two state variables, an independent t-test was used. To assess the relationship between quantitative abnormal variables and two state variables, a Mann–Whitney U test was performed.
4. Results
4.1. Hormonal Evaluation (Cortisol)
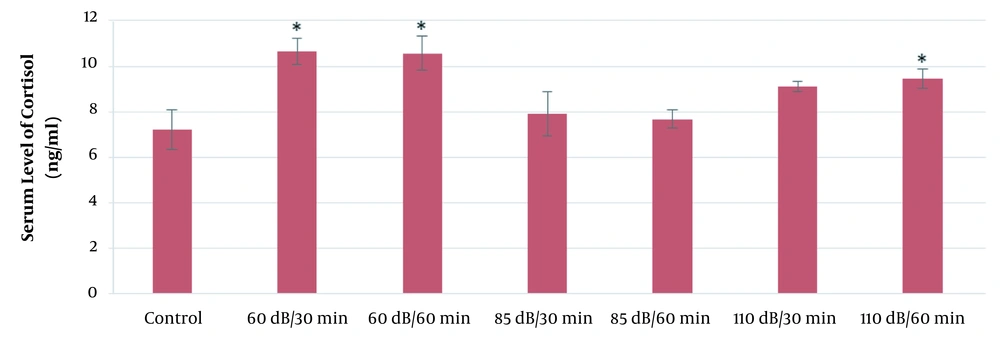
According to the results of this study, the mean serum levels of cortisol in the 110-dB (30 and 60 min), 60-dB (60 min), and 85-dB (60 min) groups were significantly higher than the control group (P ≤ 0.05). Also, the mean serum levels of cortisol in the 65-dB and 85-dB (30 min) groups were insignificantly higher than the control group (P > 0.05). The results of the effect of exposure time and noise intensity on cortisol secretion are shown in Figure 2.
As Figure 2 shows, the highest secretion of cortisol is in 110-dB (60 min) group. Also, in all exposure groups, cortisol levels increased compared to the control group. In general, cortisol secretion increased with increasing sound level and exposure time.
4.2. Hormonal Evaluation (T3, T4, and TSH)
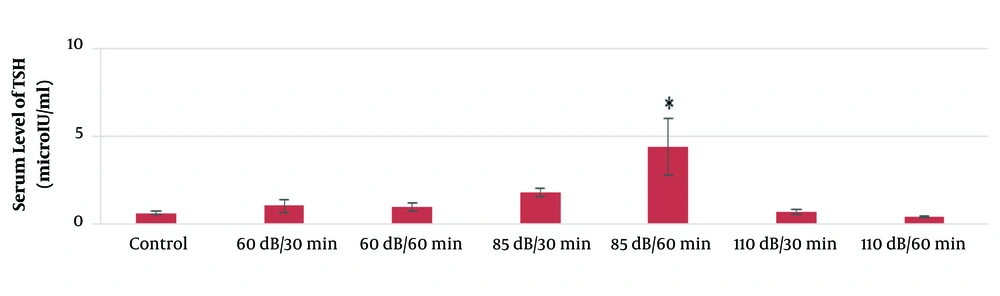
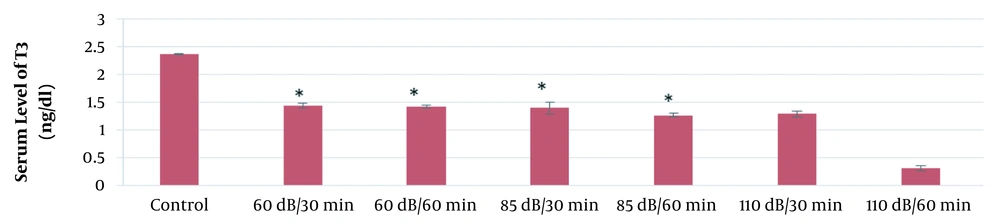
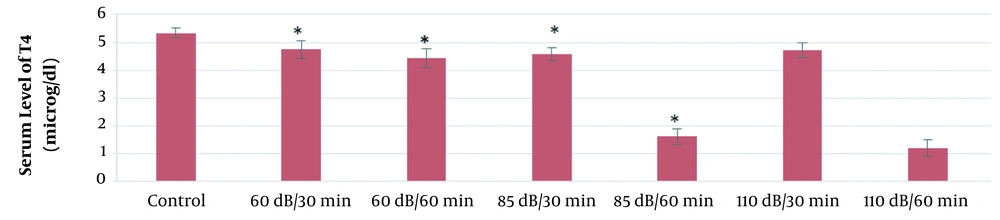
According to the results of this study, the mean serum levels of T3, T4, and TSH in the 60-dB and 85-dB groups were significantly lower than in the control group (P ≤ 0.05). However, the mean serum levels of T3, T4, and TSH in the 110-dB group were insignificantly lower than the control group (P > 0.05). The results of the effect of exposure time and noise intensity on T4, T3, and TSH secretion are shown in Figures 3 - 5.
Based on the results of the present study, the lowest secretion of mentioned hormones was in 110-dB (60 min) group, and the secretion rate of T3, T4, and TSH hormones decreased with the increase of sound level and exposure time.
4.3. Hormonal Evaluation (Testosterone)
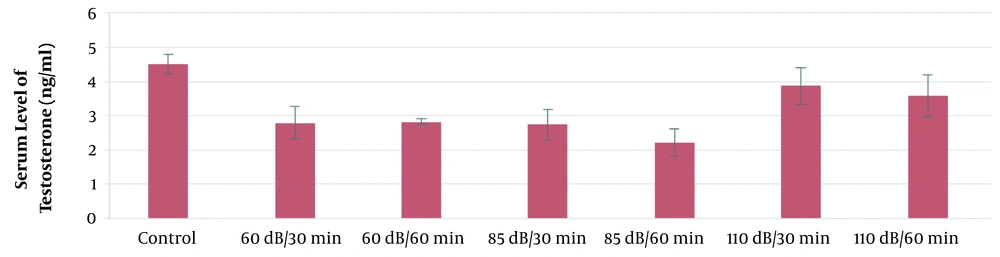
According to the results of this study, the mean serum level of testosterone in the 110-dB group was significantly higher than the control group (P ≤ 0.05). However, the mean serum levels of testosterone in the 65-dB and 85-dB groups were insignificantly lower than the control group (P > 0.05). The results of the effect of exposure time and noise intensity on testosterone secretion are shown in Figure 6.
5. Discussion
Currently, noise pollution is one of the most common environmental pollutants with direct and indirect effects on humans. Noise has adverse impacts on most body organs (29, 30). The results of this study revealed that exposure to noise raised cortisol secretion levels in rats. Cortisol level raised with increasing the exposure time and noise levels. The studies conducted by Farzadinia et al. (26) and Smitha and Mukkadan (31) showed that noise increased cortisol levels in rats, which is consistent with the results of the current study. The results of Taban et al. also showed that exposure to noise higher than 90 dB causes increased glucose and cortisol in male rats (32). Although we cannot directly compare the results of laboratory animal studies with human studies, it is important to note that cortisol is commonly recognized as a stress hormone. Generally, high levels of cortisol and prolonged cortisol levels in the bloodstream (as in chronic stress) have negative effects such as cognitive impairment, thyroid dysfunction, blood sugar imbalances, a decline in immune function, and increased abdominal fat storage. Fouladi Dehaghi et al. assessed noise-induced stress by measuring salivary cortisol in 200 male participants (100 industrial workers and 100 office workers) and showed that exposure to noise higher than 80 dB in the industry had a significant effect on cortisol levels (33). In a review study, Ising et al. confirmed that even when sleeping, sound caused by aircraft and heavy vehicles could create stress in the human body (34). Another study conducted by Yaghoubi et al. indicated a significant correlation between annoyance and cortisol secretion level after noise exposure (35). Accordingly, it is suggested that this relationship be investigated in human studies as well.
The results of the present study indicated that the mean serum levels of T3, T4, and TSH in all noise-exposed groups were significantly lower than in the control group. Chamkori et al. indicated that noise pollution significantly decreased the testosterone, prolactin, LH, FSH, and thyroid hormones, and significantly increased the concentration of cortisol compared to the control group (30). Another study by El-Etreby et al. revealed that noise caused a significant decrease in TSH, which is consistent with the results of the current study (36). The study by Ray et al. showed that thyroid activity of rats reduced when they were exposed to chronic noise. This study showed that the changes in these hormones were dependent on sound dosage (37). Also, the study conducted by Mohammadi et al. showed that exposure to sound significantly reduced the level of T3 and T4 in rats (38), which is consistent with the results of the current study. However, in the study conducted by Helal et al., noise and crowding stresses caused a significant increase only in T3 and T4, while there was a significant decrease in TSH. The study was performed on female Albino rats (39).
Due to the very complicated interactions between the endocrine glands and the central nervous system, and the musculoskeletal system, impaired functioning of the glands can cause many symptoms in the organs of the body and disrupt their function (34, 40, 41). Symptoms of hypothyroidism include muscle weakness, cold fever, constipation, shortness of breath, depression, mental disorders, stomach ache, enlarged stomach, delayed body growth, hearing impairment, learning disorder, delayed speech and language development, motor speech disorders, and speech impairment (42).
According to the results of the present study, the mean serum concentration of testosterone in the experimental groups decreased compared to the control group. Farzadinia et al. exposed 40 rats to 95-dB, 105-dB, and 115-dB noise levels and reported that the sound reduced testosterone and increased cortisol levels (26). Also, the study by Yu et al. showed that, with increased noise levels, testosterone levels in rats decreased compared to the control group (43), which is consistent with the results of the current study.
Based on the results of several studies, decreased testosterone concentrations have adverse effects on ejaculation and sperm quality, as well as reduced sperm volume in the epididymis (44, 45).
5.1. Conclusion
Based on the results of this study, exposure to noise pollution enhanced the serum level of cortisol and decreased the serum levels of T4, T3, TSH, and testosterone in rats. Yet, animal studies may not predict human reactions. Bearing in mind the undesirable effects of hormonal imbalance on human physical and psychosocial status, further studies are recommended on the adverse consequences of hormonal changes caused by noise pollution. Also, more studies need to be conducted on persistent changes in hormonal levels caused by sound exposure.