1. Background
Blood-borne pathogens such as hepatitis B (HBV), hepatitis C virus (HCV) and Human immune deficiency virus (HIV) are major health problems in the world, especially in underdeveloped countries (1, 2). Despite the biological variations and differences in the pattern of infection, these viruses have mutual spreading pathways and parallel risk factors (3, 4). Viral hepatitis is globally considered as a principal challenge for health systems. In general, there are five kinds of hepatitis viruses ranging from A to E, among which HBV and HCV infections are the most serious in terms of morbidity and mortality (5). Although there are preventive vaccinations for HBV, approximately two billion individuals are subjected to the hepatitis B virus (HBV), more than 350 million of whom are infected with chronic HBV worldwide (6-8). Furthermore, HCV infection is mostly chronic, and it is estimated that about 170 million people infected with HCV around the world (9). The center and east of Asia account for the most infected areas with HCV due to the risky injections and further medical actions (7, 10). As far as HIV infection is concerned, Asian countries such as Thailand are suffering from the prevalence of HIV due to a lack of proper prevention, treatment, and public awareness (11). Recent studies reported that approximately 36 million individuals in the world have HIV (12).
Afghanistan is one of the most underprivileged countries, which is deprived of a standard health system due to 30 years of warfare and political instability, a collapsed economy, natural disasters, majority rural setting, increasing unemployment and addicted people, drug trafficking, as well as inappropriate sanitary conditions (13). Besides, Afghan refugees may have perilous behaviors during their stay in other countries, which increased the risk of viral transfection. Therefore, Afghans are at higher risk of blood-borne infections, but data about the prevalence of these infections are scarce. Epidemiological studies are fundamental to apply proper strategies for the prevention and management of blood-borne diseases. There are partial epidemiological records regarding infectious diseases in this country; the majority of the present statistics originate from specific groups, such as injecting drug users, sex employees, and intrapartum women (14-17). In Afghanistan, there has not been a standard supervision and a national survey to mirror the load of viral hepatitis (HBV and HCV) and HIV among the broad-spectrum population. Besides the epidemiologic data, access to the accurate analytical methods for screening, diagnosis, and follow up is fundamental for the proper management of viral infections. Due to all the above-mentioned limitations and problems in Afghanistan, most laboratories use a broad range of rapid commercial tests for the screening and diagnosis of viral infections while there is no data about the sensitivity and specificity of such tests (18).
2. Objectives
Given the recent advances in the management of transfection and the treatment of blood-borne diseases especially in case of HCV (19-21) as well as the lack of data about the prevalence and accuracy of diagnostic methods for these infections in Afghanistan, the present study aimed to evaluate the seroprevalence of HBV, HCV and HIV viral infections in a large group of Afghans visiting the main laboratory of Kabul, the capital of Afghanistan.
3. Methods
3.1. Sample Collection
The present cross-sectional survey was performed between April 2014 and August 2017. The subjects of the study were 196516 Afghani citizens who went Fateme-al-Zahra clinic to undergo the obligatory checkup test for traveling to Iran. This study was approved by the Academic Council of Faculty of Pharmacy (no.: 124, 12 01. 2014), Academic Council of Kabul University (no.: 114 17. 02 2014) and Research Committee General Directorate of Academic Affairs Ministry of Higher Education of Afghanistan (no.: 010106-30.03. 2014). All the subjects were asked to sign the informed consent. After that, 5 milliliters of venous blood taken and serum was separated by expert technicians and stored at 4°C for the analysis of seropositivity in HBV, HCV, and HIV.
3.2. Detection of HBV, HCV and HIV Exposure
3.2.1. Rapid Testing
Primarily tests were conducted for all samples for the detection of HBs-Ag, anti-HCV, and anti-HIV on the same day with rapid commercial kits (Abon Biopharm, Hangzhou, China). As the manufacturer claimed, the relative sensitivity, specificity, and accuracy was > 99.0%, > 96.7% and > 98.3%, respectively. According to the kit instruction, one drop (for HIV and HCV testing) or three drops (for HBV testing) of serum samples were put on the cassettes and the results were recorded after 15 minutes and then interpreted according to kits’ references.
3.2.2. The Enzyme-Linked Immunosorbent Assay (ELISA) Testing
Samples were checked by commercial enzyme-linked immunosorbent assay (ELISA) kits (Monobind, USA). The ELISA tests were performed with Dynex DS2 fully automated ELISA apparatus. In case of any discrepancy between the result of rapid test and ELISA, ELISA’s results considered as the final reports.
3.3. Statistical Analysis
Descriptive statistics such as number, percent, mean, standard deviation were generated for participants and compared between the groups using chi-square tests, as appropriate. The statistical analysis was performed with SPSS 16.0 (SPSS Inc, Chicago, IL, USA), and statistical significance was defined as a P value < 0.05.
4. Results
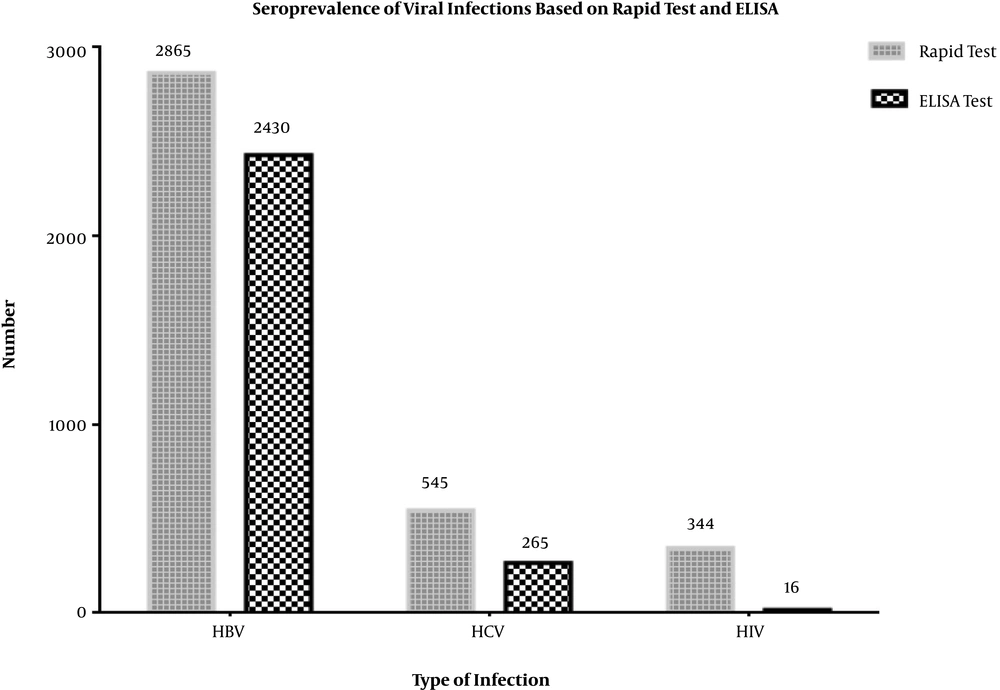
Of 196516 participants, 78% (n = 153763) were males and 22% (n = 42753) were females with mean age of 38.2 ± 15.3 (range = 4 - 90) years. The prevalence of HBs-Ag seropositivity was 1.46% and 1.23% with rapid test and ELISA, respectively (Figure 1). The positive predictive value of HBV rapid test was 84.8% compared to the confirmatory ELISA test. Unlike the various age groups (P < 0.001; Table 2), there was no significant difference in the prevalence of HBV between males and females (P = 0.15; Table 1).
| Type of infection | Total (N = 196516) | Male (N = 153763) | Female (N = 42753) | P valueb |
|---|---|---|---|---|
| HBV | 2430 (1.23) | 1920 (1.24) | 510 (1.19) | 0.15 |
| HCV | 265 (0.13) | 191 (0.12) | 74 (0.17) | 0.02 |
| HIV | 16 (0.008) | 16 (0.014) | 0 (0) | 0.05 |
aValues are expressed as No. (%).
bBy using chi-square test.
| Age Group. y | Total Subjects | Type of Infection | ||
|---|---|---|---|---|
| HBV | HCV | HIV | ||
| 1 - 10 | ||||
| Total | 13111 (6.7) | 16 (0.12) | 1 (0.008) | 0 (0) |
| Male | 7227 (55.1) | 14 (0.10) | 1 (0.008) | 0 (0) |
| Female | 5884 (44.9) | 2 (0.02) | 0 (0) | 0 (0) |
| 11 - 20 | ||||
| Total | 19137 (9.7) | 195 (1.02) | 5 (0.026) | 0 (0) |
| Male | 12609 (65.9) | 142 (0.74) | 4 (0.020) | 0 (0) |
| Female | 6528 (34.1) | 53 (0.28) | 1 (0.006) | 0 (0) |
| 21 - 30 | ||||
| Total | 80071 (40.7) | 742 (0.93) | 55 (0.068) | 4 (0.005) |
| Male | 68578 (85.6) | 646 (0.81) | 48 (0.060) | 4 (0.005) |
| Female | 11493 (14.4) | 96 (0.12) | 7 (0.008) | 0 (0) |
| 31 - 40 | ||||
| Total | 40080 (20.4) | 468 (1.17) | 59 (0.15) | 6 (0.015) |
| Male | 33828 (84.4) | 402 (1.00) | 41 (0.10) | 6 (0.015) |
| Female | 6252 (15.6) | 66 (0.17) | 18 (0.05) | 0 (0) |
| 41 - 50 | ||||
| Total | 22955 (11.7) | 412 (1.79) | 61 (0.026) | 4 (0.017) |
| Male | 17990 (78.4) | 311 (1.35) | 40 (0.17) | 4 (0.017) |
| Female | 4965 (21.6) | 101 (0.44) | 21 (0.091) | 0 (0) |
| 51 - 60 | ||||
| Total | 12071 (6.1) | 339 (2.81) | 51 (0.42) | 2 (0.016) |
| Male | 7842 (65.0) | 226 (1.87) | 33 (0.27) | 2 (0.016) |
| Female | 4229 (35.0) | 113 (0.94) | 18 (0.15) | 0 (0) |
| 61 - 70 | ||||
| Total | 5682 (2.9) | 193 (3.39) | 26 (0.46) | 0 (0) |
| Male | 3383 (56.5) | 131 (2.30) | 18 (0.32) | 0 (0) |
| Female | 2299 (43.5) | 62 (1.09) | 8 (0.14) | 0 (0) |
| > 71 | ||||
| Total | 3409 (1.7) | 65 (1.9) | 7 (0.20) | 0 (0) |
| Male | 2306 (67.6) | 48 (1.40) | 6 (0.18) | 0 (0) |
| Female | 1103 (32.4) | 17 (0.50) | 1 (0.02) | 0 (0) |
| Total | ||||
| Total | 196516 (100) | 2430 (1.23) | 265 (0.134) | 16 (0.008) |
| Male | 153763 (78.3) | 1920 (0.98) | 191 (0.097) | 16 (0.008) |
| Female | 42753 (21.7) | 510 (0.26) | 74 (0.037) | 0 (0) |
| P value | < 0.001 | < 0.001 | 0.57 | |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immune deficiency virus.
aValues are expressed as No. (%).
bby using chi-square test.
In the case of HCV, the seroprevalence of anti-HCV antibody was 0.28% and 0.13% with rapid test and ELISA, respectively (Figure 1). The positive predictive value of HCV on rapid test comparing to confirmatory ELISA test was 48.6%. The rate of HCV seroprevalence was significantly higher in females (0.17% vs. 0.12%, P = 0.02). In addition, the prevalence of anti-HCV in different age groups was statistically significant (P < 0.001) with a higher rate among the 61 - 70 years’ group (Table 2).
The prevalence of anti-HIV antibody was 0.17% and 0.008% by rapid test and ELISA, respectively. The positive predictive value of the HIV rapid test was 4.6% comparing to confirmatory ELISA test. The HIV infection was observed only in men (Table 1), but the distribution of anti-HIV was not statistically significant based on different age groups (Table 2).
Regarding co-infection, among 196516 individuals, 10 individuals (0.005%) had two infections; 9 individuals (0.004%) simultaneously had HCV and HBV and 1 subject (0.0005%) infected with both HIV and HBV.
5. Discussion
Afghanistan has been a war-stricken country with several health issues, so obtaining correct information about the prevalence and burden of diseases is the first step for proper planning. Blood-borne infections are among the major health problems with their direct and indirect costs. In the present study, we checked the prevalence of the three most important viral infections in the largest sample study performed in Afghanistan. There is scarce epidemiologic data about the prevalence of blood-borne infections in this country. A few studies have mentioned the importance of HBV, HCV, and HIV in the high-risk individuals, including drug-injecting users, blood donors, sex workers and Afghan refugees who do not reflect the total population (15, 22, 23). To the best our knowledge, this is the first study on a large sample population in Afghanistan and even in the world which might reflect the real status of this country.
Concerning the HBV infection, our target population showed a prevalence rate of 1.23% identified higher in males than females (1.24% vs. 1.19%). The HBV infection is mostly common in the group of 60 - 70 years of age compared to other age groups. In a recent comprehensive study, the global prevalence of HBs-Ag was reported 3.9% (24). The restricted epidemiological records on these infectious illnesses have been achieved either from definite study groups or from Afghan individuals who immigrated to neighboring countries. In the recent study on 1231 adult citizens of Mazar-e-Sharif City, the frequency of HBV was 5.6% and 5.4% for HBs-Ag on rapid test and ELISA, respectively which is quite higher than our study. The considerable risk factor for HBV infections was jaundice history and blood transfusion (25). Serology testing among 350 pediatric with mean age of 6.5 ± 4.2 at Kabul showed that the rate of positivity for HBs-Ag was 3.6% (n = 12) (26). Our study showed that 0.65% and 8% of the HBV positivity belonged to the group of 1 - 10 and 11 - 20 years of age, respectively. Another study in Kabul by two serum-based rapid tests reported that among 4452 intrapartum women, the frequency of HBs-Ag was 1.53% which is in consistent with our result (17).
Moreover, a study was conducted in Pakistan on 3679 blood donors during 2016 - 2017 in which the samples were screened through the ELISA kit. In agreement with our data, this study showed that HBV prevalence among the target population was 1.4% (n = 54) (27). Another study in Southern India was performed on the seventy five camps across 14 districts of Tamil Nadu from the beginning of 2014 till July of 2017. Screening was carried out by rapid assays and confirmed by ELISA. Of 18589 individuals, 1.63% (n = 303) were detected with HBV infection in the target population which is compatible with the present data (28). Consistently, in a study among 5235 Iranian subjects aged 15 - 70 years, the prevalence rate of HBs-Ag was 1.6% (n = 85).
Given the onset of the HBV vaccination program in 2006, a lower prevalence of HBV is expected among 1 - 10 years of age group (29), but the current study showed a lower prevalence of HBV comparing to other studies in Afghanistan. This discrepancy can be explained by the fact that people who are planning to travel to Iran are aware of the screening program, so those with known viral infections or high-risk behavior possibly do not apply to travel to Iran.
In the case of HCV infection, our target population showed the prevalence rate of 0.13% was significantly higher in females than males (0.17% vs. 0.12%). Several recent studies reported the global prevalence of 1% for HCV that was higher than our result data with the overall frequency of 0.13% in both genders (30-32). In one study conducted among a sample of the Afghan National Army, the distribution of HCV was 0.82%, which was higher than our finding (33). In their study, HCV reactive rapid analysis underwent the confirmatory assay through PCR conducted as the primary confirmatory examination for HCV. Furthermore, a recent report on the citizens of Mazar-e-Sharif city showed that 0.2% (n = 3) of them were sero-positive for anti-HCV on rapid tests (25). Another study among intrapartum patients in Kabul, Afghanistan, the anti-HCV positivity was reported to be 0.31% (17). Further study conducted in Kabul showed that the rate of seropositivity for HCV among children aged 6 to 15 years was 1.2% which was lower than our prevalence in children aged between 1 - 20 years (2.3%) (26). In Pakistan, the prevalence of HCV among 3679 blood donors screened through the ELISA kit was 0.6% (27). Another neighboring country showed a seroprevalence of 0.3% for HCV among a large number of individuals that was higher than our of finding (0.13%) (28). In a recent meta-analysis, the overall seroprevalence of HCV was estimated ranging from 0.08% - 1.6% in different provinces of Iran (34). Also, another meta-analysis from Iran, declared that the total frequency of anti-HCV was 0.3%; however, the rate increased to 6.2% and 32.1% for intermediate and high-risk populations, respectively (35). In another survey from Iran, the prevalence of HCV infection was 0.2% and 0.14% by ELISA and PCR, respectively (36).
As far as HIV infection is concerned, our target population showed a prevalence rate of 0.008% that was identified absolutely in men, and the highest rate belonged to the group of 41 - 50 years of age. The result of Tanju et al. (26) was compatible with ours, as they found no viral frequency in the group age of 0 - 20 years in both genders at Kabul. They indicated that probable risk factors for the transmission of viral viruses in childhood are restricted and may increase with age. Further studies on a sample of Afghan National Army aged 18 - 35 years, showed the frequency rate of 0.063% for HIV infection that was higher than our finding (0.02%) (33). Inconsistent pieces of evidence showed that none of the children and adult participants had HIV seropositivity in Kabul and Mazar-e-Sharif cities (28, 37). Another study on the sex workers in three different cities of Afghanistan (Kabul, Jalalabad and Mazar-e-Sharif) reported the prevalence of 0.19% for HIV infection (23). Moreover, in Kabul, anti-HIV positivity was not detected among the intrapartum patients (17).
Furthermore, one study in Pakistan reported the prevalence rate of 0.1% for HIV among blood donors, which was higher than our result (27). Another neighboring country evaluated the frequency of HIV infection in Afghan refugees in northeast of Tehran, Iran, through a rapid test. It showed a higher distribution of HIV infection comparing to ours (0.2% vs. 0.008%) (38). One possible reason for the difference in the results is that people with risky behaviors are unlikely to apply for a trip to other countries as they are aware of obligatory medical examinations. However, many of them donate blood just to get the result of the tests which may increase the prevalence of detected viral infections among blood donors (39).
Also, there is a major concern about the sensitivity and specificity of rapid tests. Rapid Diagnostic testing (RDT) systems are available to detect different antigens or antibodies, such as HIV-ab, HBs-Ag, and HCV-ab. They are used in different parts of the world, particularly for primary screening. Despite the high sensitivity and specificity, studies have shown that there is a great variation in their sensitivity and specificity. In a study by Robert J. O’Connell, the sensitivity of some RDTs, such as instant view cassette, FirstVue HCV, and CORE HCV, was around 55%, 64% and 35%, respectively which is somewhat low (40). Moreover, the comparison of the sensitivity of the rapid test and ELISA for the detection of HCV in Cameron confirmed the significant higher sensitivity of ELISA test than Rapid test (91.9% Vs. 70.3% (41). In contrast, another study reported higher than 95% sensitivity and specificity in detecting HBs-Ag for several commercial rapid tests (42).
5.1. Limitation
Despite the large sample size and using the ELISA test for confirmation of positive results, we encountered a few limitations in this study. For instance, we had to examine those subjects who decided to travel to Iran. This may have caused a selection bias as infected people, or people with risky behaviors possibly do not apply for this trip. Also, the sensitivity and specificity of ELISA were usually higher than the rapid test, but the rapid test was more cost-effective and feasible considering the socio-economic situation in Afghanistan.
5.2. Conclusions
To the best of our knowledge, this was the largest study in Afghanistan whose results showed a rather low prevalence of HBV, HCV, and HIV, especially among children and women. Given the selection bias, the real prevalence of blood-borne infections was expected to be much higher. By comparing the result of both HCV and HIV with rapid test and ELISA, the ELISA method is strongly recommended. Regarding the prevalence of HBV and HCV, taking proper strategy for public awareness, screening and treatment is an urgent need.