1. Background
The novel Coronavirus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has emerged from Wuhan, China, in December 2019 and it was declared as a pandemic by the World Health Organization (WHO). In a very short period, many countries, including Iran, have faced this important health challenge. Belonging to the Coronaviridae family, SARS-CoV-2 is an enveloped positive-sense RNA virus. Middle East Respiratory Syndrome-related Coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) from this family were previously responsible for respiratory tract infection outbreaks in 2012 and 2002, respectively (1, 2). Despite the lower mortality rate of SARS-CoV-2 compared to other family members, high contagion and the ability to persist on surfaces from hours to days have made global health policy-makers to pay particular attention to COVID-19 (3). Since the emergence of the virus, several studies have been published on the association of some blood biomarkers with COVID-19 infection such as leukopenia (4), elevated high-sensitivity C-reactive protein levels, elevated Erythrocyte Sedimentation Rate (ESR) (5), and abnormal liver enzymes. It has also been reported that SARS-CoV-2 can affect liver cells by causing elevated levels of aminotransferase enzymes and liver dysfunction (6). Another study identified no viral particle in the liver biopsy of a MERS-CoV patient, but portal inflammation, perivenular necroinflammation, loss of hepatocytes, and normal liver enzyme values were observed (7).
In COVID-19 patients, the abnormalities of liver enzymes have been reported to range from 20% to 50%, but the relevance of this finding to the prognosis or progression of the disease is still controversial (8, 9).
2. Objectives
This study aimed to evaluate the liver enzyme changes in COVID-19 patients and any possible association with prognosis.
3. Methods
3.1. Study Population
This cross-sectional study was performed on patients admitted to referral hospitals of Mazandaran University of Medical Sciences from February to March 2020. The diagnosis was made base on clinical symptoms, Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR), and chest Computed Tomography (CT) scan by expert infectious and internal specialists. Patients with viral liver infections, cirrhosis, hepatocellular carcinoma, alcohol abuse, and patients whose disease did not require hospitalization were excluded from the study. A group of healthy people as the control group was enrolled from the Tabari Cohort population matched to the age and sex of patients. Further details concerning this Cohort has been described in the cohort profile (10). A convenience sampling method was used for the case group. In the control group, 186 persons were selected from 10,255 persons by simple random sampling and software based on the matching rule.
3.2. Subject Evaluation
For patients in the main group, complete blood count (Celltac Es MEK-7300 hematology automatic analyzer, Nihon Kohden, Japan), liver enzymes (Selectra pro xl Clinical Chemistry System, The Netherlands), qualitative C-reactive protein (Enison Lab and Pharmaceutical Industries, Tehran, Iran), and erythrocyte sedimentation rate (Sedimex, Parsian Teb medical, Tehran, Iran) were ordered by the physician as part of the diagnosis-treatment program at the time of admission before prescribing any medication for COVID-19. Therefore, no additional sampling was performed for the study. Normal laboratory values were as follows: lymphocyte (absolute count): 1,000 - 4,000 per mm3, platelet: 150,000 - 450,000 per mm3, AST: up to 40 U/L, ALT: up to 45 U/L in males and up to 34 U/L in females, ALP: up to 270 U/L in males and up to 240 U/L in females, total bilirubin: 0.3 - 1.2 mg/dL, direct bilirubin: up to 0.3 mg/dL, LDH: up to 470 U/L, ESR: up to 15 and 20 in males under and over 50 years, and up to 20 and 30 in females under and over 50 years, respectively. The length of hospital stay, intensive/critical care requirement, and mortality were considered as prognostic indicators. Clinical and epidemiologic data were extracted from the medical records.
3.3. Ethical Consideration
The study obtained ethical approval from the Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1398.7296).
3.4. Statistical Analysis
Data were analyzed by SPSS software (version 20). The t-test was used for the comparison of baseline characteristics in both groups, the partial correlation for the relationship of liver enzymes and bilirubin with inpatient days, linear regression for the relationship between admission days and quantitative variables, logistic regression for the relationship of intensive care and mortality with qualitative variables, and Receiver Operating Characteristic (ROC) curve for sensitivity and specificity of AST levels. We use the enter method in both linear and logistic regression models. To investigate the normal distribution of quantitative variables, histogram plots were used. A P < 0.05 was regarded as the level of significance.
4. Results
This study was performed on 93 patients with COVID-19 and 186 people from the Tabari Cohort normal population as the control group. The case/control ratio was 1/2, and the matching for sex and age was at the individual and age-group levels, respectively. The mean age of the patients and controls was 56.3 ± 15.2 and 54.1 ± 11 years, respectively (P = 0.228). We matched for the gender variable, so there were 42 (45.2%) female subjects and 51 (54.8%) male subjects in both groups. Underlying diseases were found in 45 (48.3%) patients, as follows: diabetes (16 patients, 17.2%), hypertension (10 patients, 10.7%), history of cardiovascular disease (four patients, 4.3%), cancer history (four patients, 4.3%), and asthma (two patients, 2.1%), in sequence.
The comparison of the two groups for laboratory parameters showed that the numbers of lymphocytes and platelets were significantly lower in patients than in controls. Also, AST, ALT, and ALP values were higher in patients than in controls (Table 1). The most common hepatic impairment events were increased direct bilirubin (45.8%), ALT (30.3%), AST (29.2%), ALP (17%), and total bilirubin (10.2%), in sequence. The mean length of hospital stay was 6 ± 2.3 days, with 36 (38.7%) patients requiring intensive and critical care and 17 (18.3%) patients being expired. Based on the partial correlation analysis controlled for confounding variables including lymphopenia, thrombocytopenia, positive CRP, elevated ESR, there was no association between the levels of AST (r = 0.032, P = 0.831), ALT (r = 0.008, P = 0.956), ALP (r = -0.077, P = 0.610), total bilirubin (r = 0.007, P = 0.961), and direct bilirubin (r = 0.033, P = 0.828) and the length of stay in the hospital.
| Group | Na | Mean | P Value |
|---|---|---|---|
| Age, y | 0.228 | ||
| Control | 186 | 54.1 ± 11 | |
| Case | 93 | 56.3 ± 15.2 | |
| BMI, kg/m2 | 0.30 | ||
| Control | 186 | 27.6 ± 4.5 | |
| Case | 66 | 27 ± 3.4 | |
| WBC, per mm3 | 0.473 | ||
| Control | 186 | 6553.6 ± 1650.9 | |
| Case | 93 | 6789.7 ± 2936.4 | |
| Lymphocyte, per mm3 | < 0.001 | ||
| Control | 186 | 2465.1 ± 796.6 | |
| Case | 93 | 880.6 ± 527.9 | |
| Hemoglobin, g/dL | 0.421 | ||
| Control | 186 | 14.1 ± 1.6 | |
| Case | 87 | 13.2 ± 10.4 | |
| Platelet, per mm3 | < 0.001 | ||
| Control | 186 | 255.2 ± 63.8 | |
| Case | 86 | 209.4 ± 62.7 | |
| AST, U/L | < 0.001 | ||
| Control | 186 | 19.9 ± 7.5 | |
| Case | 89 | 39.5 ± 34.9 | |
| ALT, U/L | < 0.001 | ||
| Control | 186 | 21.6 ± 12.7 | |
| Case | 89 | 40.4 ± 46.5 | |
| ALP, U/L | 0.004 | ||
| Control | 186 | 222.2 ± 70.6 | |
| Case | 88 | 192.6 ± 91.2 | |
| Total Bilirubin, mg/dLb | |||
| Case | 59 | 0.7 ± 0.6 | |
| Direct Bilirubin, mg/dLb | |||
| Case | 59 | 0.4 ± 0.3 | |
| LDH, U/Lb | |||
| Case | 74 | 575.8 ± 378.5 | |
| ESR, mm/hb | |||
| Case | 81 | 41 ± 19.7 |
Baseline Characteristics of Study Populations (Mean ± SD)
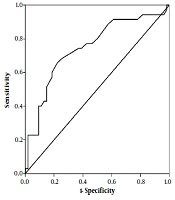
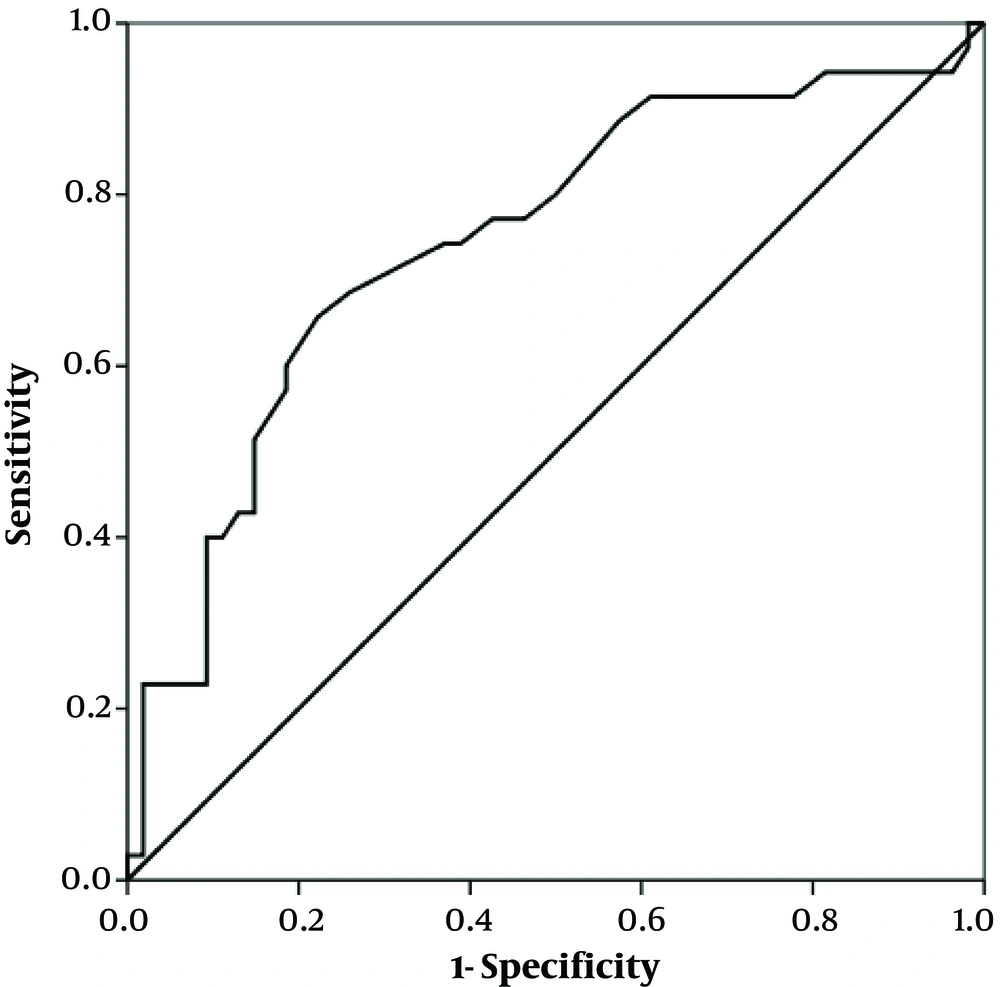
According to logistic regression, the risk of being transferred to the critical/intensive care unit was strongly associated with the elevated levels of AST and direct bilirubin (OR = 97.08 and OR = 7.99, respectively) (Table 2). The ROC curve was also plotted where the area under the curve was 0.75 (95% CI: 0.64 - 0.85, P ≤ 0.001), and AST = 30.5 (U/L) had a sensitivity of 71.4% and specificity of 68.5% for critical/intensive care transfer (Figure 1). It was also observed that the mortality rate significantly increased with increased AST levels (OR = 50.54) (Table 3).
| Variable | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| AST (elevated) | 97.08 | 3.14 | 2993.97 | 0.009 |
| ALT (elevated) | 0.72 | 0.04 | 13.18 | 0.827 |
| ALP (elevated) | 0.19 | 0.02 | 1.71 | 0.141 |
| Total Bilirubin (elevated) | 0.75 | 0.03 | 18.27 | 0.860 |
| Direct Bilirubin (elevated) | 7.99 | 1.3 | 48.9 | 0.025 |
| Lymphopenia | 1.48 | 0.23 | 9.51 | 0.676 |
| Thrombocytopenia | 1.79 | 0.12 | 25.65 | 0.667 |
| CRP (positive) | 2.83 | 0.48 | 16.54 | 0.248 |
| Age | 1.04 | 0.98 | 1.11 | 0.125 |
| Background diseases | 0.75 | 0.16 | 3.51 | 0.720 |
| Constant | 0.004 | 0.023 | ||
Association Between AST, ALT, ALP, and Bilirubin and Critical/Intensive Care Unit Transfer
| Variable | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| AST (elevated) | 50.54 | 1.73 | 1469.75 | 0.023 |
| ALT (elevated) | 0.43 | 0.01 | 10.58 | 0.607 |
| ALP (elevated) | 0.53 | 0.05 | 5.00 | 0.579 |
| Total Bilirubin (elevated) | 0.14 | 0.003 | 6.70 | 0.319 |
| Direct Bilirubin (elevated) | 7.12 | 0.60 | 83.99 | 0.119 |
| Lymphopenia | 7.86 | 0.43 | 142.74 | 0.163 |
| Thrombocytopenia | 0.53 | 0.04 | 6.67 | 0.624 |
| CRP (positive) | 0.56 | 0.08 | 3.75 | 0.553 |
| Age | 1.05 | 0.98 | 1.12 | 0.157 |
| Background diseases | 3.52 | 0.47 | 25.87 | 0.216 |
| Constant | 0.00 | 0.009 | ||
Association Between AST, ALT, ALP, and Bilirubin and COVID-19-related Mortality
5. Discussion
We found elevated levels of liver enzymes and bilirubin in patients with COVID-19 infection in association with the need for critical/intensive care unit (AST and direct bilirubin) and mortality (AST); however, they were not correlated with the length of hospital stay. The elevated levels of blood aminotransferases can take place due to liver injury or extrahepatic conditions such as thyroid disorders, heart or muscle disease, inflammatory bowel disease, etc. Also, it should be noted that although ALT is a specific liver damage marker, AST changes are non-specific because of its widespread expression (11, 12). Several studies have reported liver function test impairment in association with Coronaviridae family infections. There are two main hypotheses in this regard: direct liver attack by the virus and systemic inflammation (2).
In an autopsy investigation of four cases with SARS-CoV infection, the virus was detected not only in the respiratory system (lung, trachea, and bronchus) but also in the digestive system (stomach, small intestine, pancreas, and liver), possibly with the origin of virus-contaminated food and water (13). As some COVID-19 patients have gastrointestinal symptoms, and viral DNA is detectable in stool samples, the fecal-oral route of transmission has been suggested, and it may be responsible for liver involvement (14, 15).
In an experimental study by Zhao et al., a transgenic mouse model expressing MERS-CoV receptor (codon-optimized human dipeptidyl peptidase 4) exhibited liver damages, including hepatocyte necrosis, fatty changes, and immune cell infiltration. However, viral antigens were expressed in lung, kidney, and brain tissues (16). In liver biopsies of three SARS patients whose ALT levels were high, RT-PCR revealed the viral infection of liver cells; also, lymphocytic infiltration, high mitotic activity, and apoptosis were observed as pathologic changes (6).
Various liver impairment degrees were reported by Chen et al. in 99 COVID-19 patients, as follows: decreased albumin levels in 98% of patients, elevated levels of ALT in 28% and AST in 35%, and total bilirubin in 18% (17). About one-third of critically ill COVID-19-infected patients displayed liver dysfunction, as reported by Yang et al. Nevertheless, the prevalence of abnormal liver tests did not differ between the survivor and non-survivor patient groups (18). However, in another study of 41 patients infected with COVID-19, liver aminotransferases, and total bilirubin were significantly higher in those requiring critical and intensive care than in others (19). It has been shown that Angiotensin-converting Enzyme 2 (ACE2) acts as an entry receptor for both SARS-CoV and SARS-CoV-2. Although 60% of cholangiocytes express ACE2, in contrast, a very small proportion of hepatocytes (2.6%) express this receptor. This may highlight that bile ducts (not the liver) may act as the target of the virus attack (20).
Also, systemic inflammation and elevated levels of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF observed during SARS-CoV-2 infection can play a role in liver enzyme disturbance (21). High CRP concentrations are associated with increased liver enzymes, too (22, 23). Liver damage can occur not only by hepatotropic viruses (hepatitis viruses) but also by systemic, respiratory infections (EBV, Influenza, and SARS-CoV) because of immune responses (24). Hepatocyte injury or hepatitis associated with non-hepatotropic infections seems to be mostly related to the cytokine storm, oxidative stress in hepatocytes, liver CD8+ T cells, and hypoxemia (25, 26).
Non-alcoholic Fatty Liver Disease (NAFLD) is a common cause of elevated liver enzymes, which is often associated with high BMI (27, 28). To overcome this issue, we used a matched BMI control group, and the subjects of this study were not in the high BMI range. Drug-induced Liver Injury (DILI) is another cause of abnormal liver enzymes. Our effort in this study was to conduct laboratory evaluations before the administration of COVID-19 therapy. Since serial laboratory evaluations were not performed, we cannot comment on the relationship between changes in these variables and prognosis. The strengths of this study include the presence of a control group, the exclusion of other diseases effective on liver enzyme tests, and the investigation of the relationship between liver enzyme tests and outcomes.
Further studies are needed to summarize hepatocellular involvement caused by COVID-19 infection. Given the undeniable role of the immune system in this phenomenon, any possible interpretation should be considered with caution.