1. Background
Liver failure, as a pivotal organ in the maintenance of homeostasis and detoxification, may bring about an increased risk of malnutrition, infection, bleeding, and reduced cognitive function according to the stage of the damage (1, 2). Both acute and chronic liver damage may also result in neuropsychiatric complications, including dysfunctions of memory, attention, and psychomotor ability, which significantly decrease the individual’s quality of life (3).
Previous studies have shown that hepatotoxic chemicals such as thioacetamide (TAA) and carbon tetrachloride (CCl4) can lead to acute and chronic liver damage both in humans and experimental animal models by inducing Reactive Oxygen Species (ROS) production and inflammatory response in the body (3-5). Increased ROS and inflammatory cytokines are considered as major mechanisms involved in toxin-induced liver damage (6, 7). These hepatotoxic agents can cause inflammation, necrosis, cirrhosis, cancer, encephalopathy, and even death, based on the extent of exposure (3-5). Hyperammonemia caused by the inability of the liver to wash out the excess amount of ammonium (NH4) is believed to play a certain role in developing Hepatic Encephalopathy (HE) that consists of several neuropsychiatric comorbidities such as memory dysfunction, anxiety, depression, progressive confusional state, and eventually coma and death (3, 8-10). Thus, one of the main goals of the treatment of hepatic failure is to reduce these neuropsychiatric complications by preventing further toxin-induced damage to liver tissue and to buy time for patients on the waiting list for a liver transplant as the definite cure (3, 8-10).
Resveratrol, a natural antioxidative and anti-inflammatory agent, has shown to possess anti-platelet, anti-cholesterol, anti-viral, anti-aging, anti-cancer, and neuroprotective effects, as well as hepatoprotective effects, both in vivo and in vitro (11-13). Several studies have investigated the hepatoprotective role of resveratrol, showing that it can inhibit the progression of hepatic diseases in various models of liver damage, including alcoholic/non-alcoholicnon-alcoholic fatty liver, ethanol-induced/dimethylnitrosamine-induced liver failure, and TAA-induced chronic liver damage (13-18). However, the effect of resveratrol on HE and neurobehavioral alterations caused by liver dysfunction has not been studied, particularly in a model of acute liver failure.
2. Objectives
To further evaluate the efficacy of resveratrol in liver dysfunction, the current study aimed to examine the effects of resveratrol on neuropsychiatric complications and liver function of rats with TAA-induced acute liver failure.
3. Methods
3.1. Study Groups
In this experimental study, 48 healthy adult male Wistar laboratory rats (200 ± 20 g) were obtained from the Animal Laboratory of Shiraz University of Medical Sciences, Shiraz, Iran. The animals were kept in standard cages at 23 ± 2ºC temperature, 40% - 45% humidity, a 12:12 h light-dark cycle, and sufficient food and water ad libitum during the experiment. The rats were divided into four groups (n = 12) randomly, as follows:
Control group 1 (C1): Receiving 1 ml of distilled water Per Oral (PO) daily
Control group 2 (C2): Receiving 1 ml of distilled water PO daily plus a single dose of TAA (350 mg/kg body weight) on day 3 of the study
Study group 1 (E1): Treated with resveratrol (5 g/kg body weight) PO daily plus a single dose of TAA (350 mg/kg body weight) on day 3 of the study
Study group 2 (E2): Receiving resveratrol (10 g/kg of body weight) PO daily plus a single dose of TAA (350 mg/kg body weight) on day 3 of the study
3.2. Treatments Preparation
Based on a pilot study, we concluded that respecting the weight and condition of rats, a single injection of TAA at 350 mg/kg body weight is sufficient to induce liver failure. Thioacetamide (Sigma-Aldrich™, MO, USA) was dissolved in normal saline and injected intraperitoneally (IP) on day 0. after 24 hours OF thioacetamide injection (day 1), all rats were injected with a 1 mL solution of 0.45% sodium chloride, 5% dextrose, and 0.2% potassium chloride to prevent hypovolemia, hypoglycemia, and hypokalemia (3). The last day of the experiment (day 10 here) was chosen the day in which at least 50% of the rats in the C2 group reach grade 4 of encephalopathy concerning neurobehavioral score. All treatments started from day 1 to the end of the study. The protocol of the present study was approved by the Institutional Research Board of Shiraz University of Medical Sciences, and all criteria of experiments, including laboratory animals, were considered.
3.3. Neurobehavioral Examinations
In this experiment, we evaluated rats every three days (days 1, 4, 7, and 10) for encephalopathy state (Table 1), locomotor activity (open field test), depression (forced swimming test), and anxiety (elevated plus arms) based on a previously published method (3). The open-field test was performed on a 50 × 50 cm2 square that was divided into 25 smaller squares (10 × 10 cm2). Elevated plus arms were also made using four arms, each with 10 cm width and 50 cm length. Open arms had no walls, and closed arms were surrounded by walls with 20 cm height. The pool for the swimming test was a circular basin with a 50 cm radius. The protocol of performing all the tests was obtained from a previous study (3). All the animals were trained for one week before TAA injection in all the tests. Each test lasted 5 min per animal. To avoid the probable bias during observations, all the tests were performed by one single-blinded observer.
| Clinical Grade | Definition |
|---|---|
| 0 | Normal behavior |
| 1 | Mild lethargy |
| 2 | Decreased motor activity, poor gesture control, diminished pain perception |
| 3 | Severe ataxia, no spontaneous righting reflex |
| 4 | No righting reflex, no reaction to pain stimuli |
Clinical Grading Scores of the Animals’ Behavior
3.4. Biochemical and Pathological Assessments
At the end of the experiment, blood samples were obtained from the rats. A series of the samples were centrifuged to separate the plasma. The plasma was sent to the laboratory (Dr. Saadati Pathobiology Laboratory, Shiraz, Iran) for measuring the ammonia level. Other series of blood samples were sent to the same laboratory for measuring aspartate aminotransferase/alanine aminotransferase (AST/ALT), alkaline phosphatase (Alk), and total bilirubin (TB) using clinical test kits (Randox™, Randox Laboratories Ltd., UK).
After sacrificing the animals, liver specimens were obtained and fixed immediately in 10% phosphate-buffered formaldehyde for two days and then were kept in 70% ethyl alcohol until being processed. Then, 5 µm-thickness slices were prepared from liver sections and stained with hematoxylin and eosin for pathological examinations. The liver specimens were examined semi-quantitatively based on an observational scoring system previously introduced by Shankar et al. for portal inflammation, focal necrosis, apoptosis, focal inflammation, confluent necrosis, periportal or periseptal interface hepatitis (piecemeal necrosis), stage of liver damage, intraparenchymal hemorrhage (IPH), and congestion ratio, performed by a single-blinded expert pathologist (19).
3.5. Data Analysis
Laboratory data are expressed as mean ± Standard Deviation (SD). Statistical analyses were performed using the Kruskal-Wallis and Mann-Whitney U tests for comparing the groups. All tests were done via SPSS software (21.0, IBM™, USA) program, and P values of ≤ 0.05 were considered statistically significant.
4. Results
The levels of the examined markers in the biochemical evaluations are shown in Table 2. Overall, the measured values of ALT, AST, and NH4 showed lower levels in the E1 and E2 groups than in the C2 group (P < 0.05). As shown in Table 2, the plasma level of NH4 was significantly lower in the C2 group compared to E2 (P = 0.006) and E1 (P = 0.011) groups. The ALT levels were significantly lower in E1 and E2 groups than in the C2 group (P = 0.011 and P = 0.006, respectively). The AST levels were significantly lower in the E2 (P = 0.006) and E1 (P = 0.011) groups than in the C2 group.
| Groups | TB (mg/dL) | AST (IU/L) | ALT (IU/L) | Alk (IU/L) | NH4 (µg/dL) |
|---|---|---|---|---|---|
| C1 | 1.60 ± 0.31 | 33.79 ± 31.33 | 22.67 ± 19.22 | 56.67 ± 42.77 | 122.03 ± 95.21 |
| C2 | 1.34 ± 0.86 | 800.67 ± 250.78b | 259.75 ± 111.46b | 90.01 ± 16.22b | 658.14 ± 72.89b |
| E1 | 1.79 ± 0.13 | 320.51 ± 94.56b, c | 108.17 ± 80.06b, c | 96.71 ± 51.12b | 345.64 ± 129.30b, c |
| E2 | 1.64 ± 0.69 | 261.17 ± 151.85b, c | 159.33 ± 82.21b, c | 69.21 ± 41.82 | 330.83 ± 21.41b, c |
Effect of Resveratrol on TB (Total Bilirubin), ALT (Alanine Aminotransferase), AST (Aspartate Aminotransferase), Alk (Alkaline Phosphatase), and NH4 (Plasma Ammonia) in Thioacetamide-induced Acute Liver Injurya
The pathological evaluation, as shown in Table 3, displayed a significant difference between the E2 group and the C2 group (untreated liver failure rats) concerning all pathological parameters including portal inflammation (P = 0.004), focal necrosis, apoptosis, and focal inflammation (P = 0.004), confluent necrosis (P = 0.003), piecemeal necrosis (P = 0.004), stage of liver damage (P = 0.003), IPH (P = 0.003), and congestion ratio (P = 0.004). The E1 group that received a lower dose of resveratrol also showed significant differences from the C2 group in the scores of focal necrosis, apoptosis, and focal inflammation (P = 0.004), piecemeal necrosis (P = 0.004), stage of liver damage (P = 0.004), IPH (P = 0.003), and congestion ratio (P = 0.004).
| Groups | Necrosis Parameters | Liver Damage Stage (Range 0 - 6) | IPH Ratio | Congestion Ratio | |||
|---|---|---|---|---|---|---|---|
| Portal Inflammation (Range 0 - 4) | Focal Necrosis, Apoptosis, and Inflammation (Range 0 - 4) | Confluent Necrosis (Range 0 - 6) | Periportal/Periseptal Interface Hepatitis (Piecemeal Necrosis) (Range 0 - 4) | ||||
| C1 group | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C2 group | 2.6 ± 0. 9 | 2.6 ± 0.89 | 4.6 ± 1.5 | 3 ± 0.7 | 5 ± 0.7 | 40% | 60% |
| E1 group | 1.8 ± 1.3 | 1.8 ± 0.5b | 3.3 ± 1.9 | 1.8 ± 1.3b | 2.3 ± 1.6b | 17% | 33% |
| E2 group | 1.3 ± 0.6b | 1.3 ± 1.1b | 2.6 ± 2.2b | 1.8 ± 1.3b | 2 ± 1.4b | 17% | 33% |
Effect of Resveratrol on Histopathological Changes of the Liver in Thioacetamide-induced Acute Liver Injurya
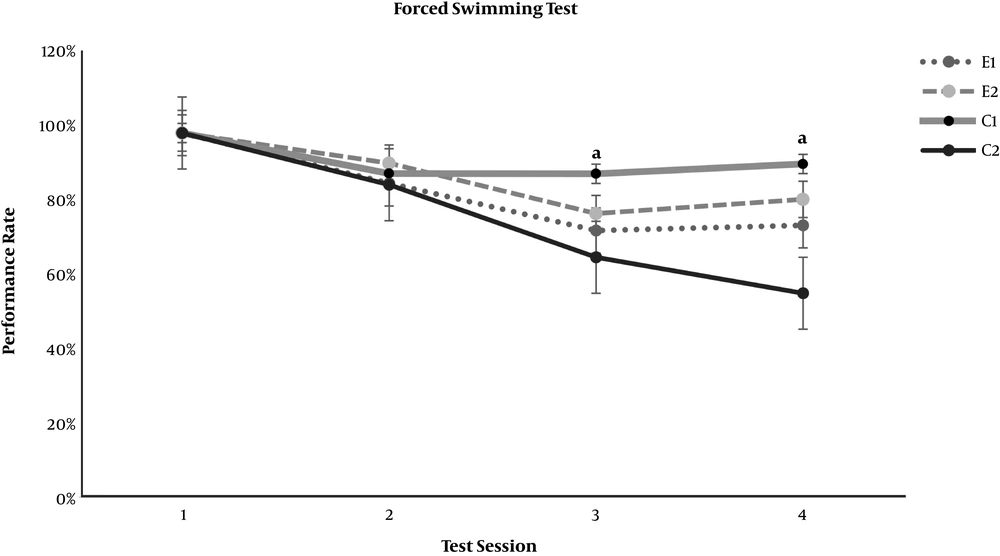
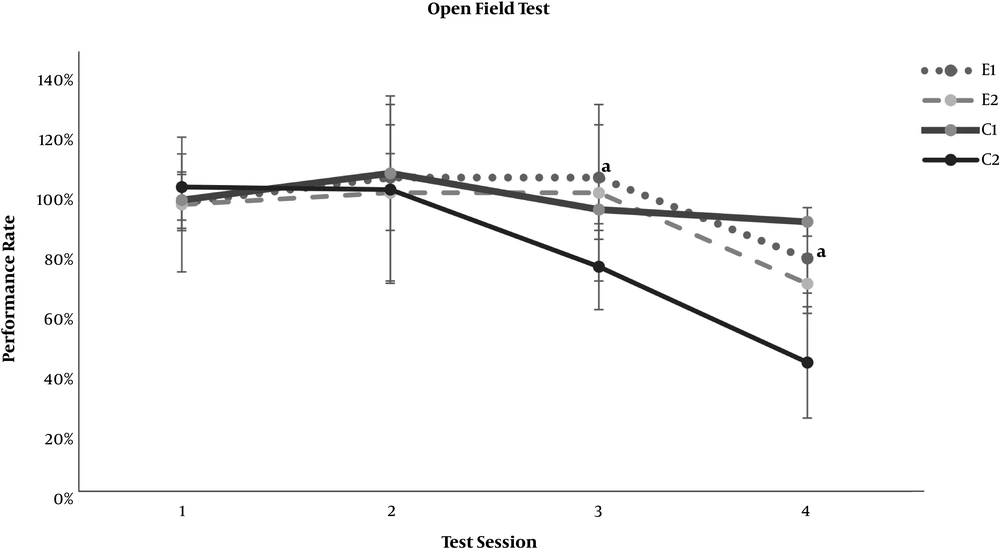
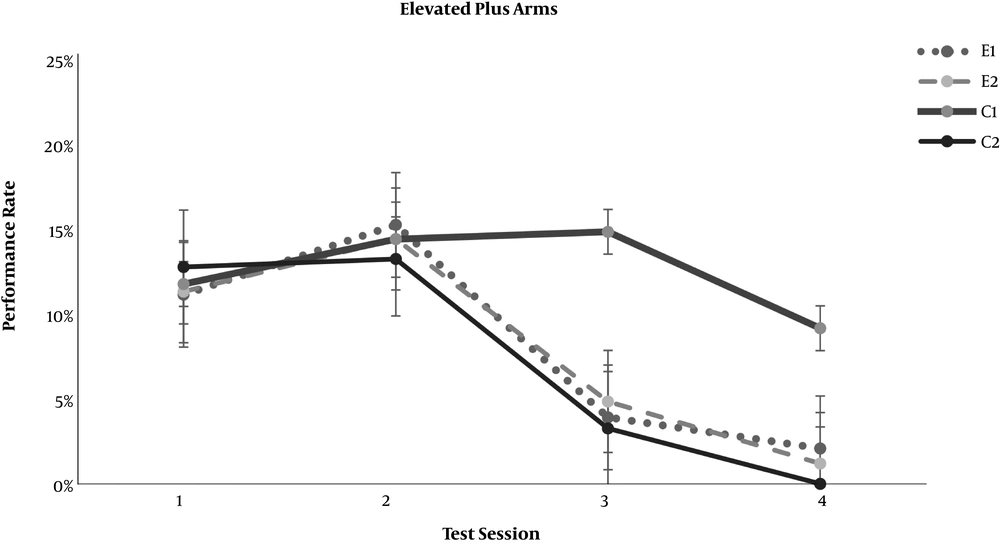
In the neurobehavioral examinations, the E1 and E2 groups showed significantly lower grades of encephalopathy than the C2 group (P = 0.020 and P = 0.011, respectively) (Table 4). As demonstrated in Figures 1 and 2, significant differences appeared between the study groups and the C2 group regarding the open-field test for motor activity and the forced swimming test for depression in the third test session (day 7 of the experiment) (P < 0.001). The anxiety level based on the elevated arms test did not show any significant difference between the C2 group and the study groups, E1 and E2 (Figure 3). On the other hand, both study groups differed significantly from the healthy C1 group regarding the open-field test, forced swimming test, and elevated arms test, starting from the third test session (P < 0.05) (Figures 1-3).
Effect of resveratrol on the forced swimming test results in thioacetamide-induced acute liver injury. Data are presented as Mean ± SD for control group 1 (C1) receiving normal saline PO daily, control group 2 (C2) receiving normal saline PO daily and a single dose of thioacetamide at 35 mg/100 mg body weight, experimental groups 1 and 2 (E1 and E2) receiving resveratrol at 0.5 and 1.0 mg per 100 mg body weight PO daily beside thioacetamide. The tests were performed on days 1, 4, 7, and 10 of the experiment. “a” shows a significant difference between the study groups and the C2 group in the correspondent session (P < 0.05).
Effect of resveratrol on the open field locomotor test results in thioacetamide-induced acute liver injury. Data are presented as Mean ± SD for control group 1 (C1) receiving normal saline PO daily, control group 2 (C2) receiving normal saline PO daily and a single dose of thioacetamide at 35 mg/100 mg body weight, experimental groups 1 and 2 (E1 and E2) receiving resveratrol at 0.5 and 1.0 mg per 100 mg body weight PO daily beside thioacetamide. The tests were performed on days 1, 4, 7, and 10 of the experiment. “a” shows a significant difference between the study groups and the C2 group in the correspondent session (P < 0.05).
Effect of resveratrol on the elevated plus arms anxiety test results in thioacetamide-induced acute liver injury. Data are presented as Mean ± SD for control group 1 (C1) receiving normal saline PO daily, control group 2 (C2) receiving normal saline PO daily and a single dose thioacetamide at 35 mg/100 mg body weight, experimental groups 1 and 2 (E1 and E2) receiving resveratrol at 0.5 and 1.0 mg per 100 mg body weight PO daily beside thioacetamide. The tests were performed on days 1, 4, 7, and 10 of the experiment.
5. Discussion
Our study showed the promising efficacy of resveratrol in TAA-induced liver failure based on serum markers, pathological evaluations, and neurobehavioral manifestations of HE. Based on the previously published literature along with the outcome of the current research, we believe that this agent, with its anti-inflammatory and antioxidant properties, can play a noticeable role as an alternative supplement in patients suffering from liver failure (11-13).
In the present investigation, laboratory parameters, including AST and ALT, as indicators of liver damage, seemed to be lower in resveratrol-treated rats than in the untreated group. It has been previously shown that both enzymes are lower in animal models of alcoholic and non-alcoholic steatohepatitis that were being treated by resveratrol (15, 16). The same results were observed in methotrexate-induced, CCl4-induced, and ethanol-induced hepatic injuries, as the resveratrol-treated subjects had lower AST and ALT levels (20, 21). Similarly, the agent has shown promising results in a clinical trial depicted by Faghihzadeh et al. in patients with NASH (17). Besides, the histopathological evaluations of liver specimens demonstrated that subjects treated with resveratrol had lower grades of portal inflammation, focal necrosis, confluent necrosis, and periseptal hepatitis, diminished liver damage stages, decreased IPH, and lower congestion, when compared to the untreated group. This pathological evaluation outcome is consistent with previous investigations that showed resveratrol supplementation could decrease steatosis, fibrosis, and necrosis in ethanol-induced liver-damaged models (16, 22). Diminished steatosis was also observed in animal models of non-alcoholic steatohepatitis (NASH) after receiving resveratrol (15). Inflammation and oxidative state were the main underlying causes of liver injuries in all these models (23, 24), and resveratrol was assumed to avert the liver damage progression by acting against these causes.
It has been noticed that patients with liver failure may present a spectrum of neurological disorders, including alterations in intellectual function and behavior, changes in consciousness, psychomotor slowing, and impairment of visuomotor and bimanual coordination (25). Considering the neurobehavioral aspect of HE, our results showed that rats treated with resveratrol had a lower grade of encephalopathy, improved motor activity, decreased depression based on the forced swimming test, and diminished anxiety according to the elevated plus-maze test. This outcome was also consistent with a previously published paper by Malaguarnera et al. reporting that resveratrol administration improved depression, anxiety, and quality of life in patients with Minimal Hepatic Encephalopathy (MHE) (26). We demonstrated that resveratrol could diminish the plasma levels of ammonia in liver-damaged animal models. Ammonia is a metabolite that seems to be associated with HE and may elevate in both acute and chronic liver failures; it can cross the blood-brain barrier and result in neurobehavioral manifestations of hepatic failure (27, 28). Previous studies have attempted several agents such as L-carnitine, quercetin, ornithine phenylacetate, glycerol phenylbutyrate, and probiotics to reduce the blood and brain concentrations of ammonia, aiming to treat HE (29-31). Ammonia remains as one of the main targets of HE treatment, and the ammonia-lowering strategies, aiming at reducing ammonia production or improving ammonia removal, have been proposed for the treatment of this condition (31, 32). Bobermin et al. demonstrated that resveratrol could attenuate the activity of ROS, thus reducing the ammonia-induced oxidative stress (27). Furthermore, it has been mentioned that resveratrol contributes to neuronal excitotoxicity prevention and ammonia detoxification (33). According to our results, this therapeutic outcome of resveratrol administration may be dose-dependent, as the models that received the higher doses of the agent had lower plasma levels of ammonia. However, this assumption should be further investigated in future studies.
This study had some limitations to be considered. Insufficient training (here for one week) of the animals before starting the experiments may result in the cognitive examinations’ bias. We did not evaluate the prothrombin time, which is another indicator of liver dysfunction, due to limitations in blood samples and funding resources. The relatively small sample size of the study is another limitation that must be mentioned, as a larger sample size could lead to more comprehensive outcomes.
5.1. Conclusion
According to the results of the present study, resveratrol supplementation can delay the progression of hepatic damage and its neurobehavioral manifestations. Moreover, the current evidence is mostly in favor of prescribing resveratrol as an alternative treatment or supplement. To the best of our knowledge, no serious side effect has been reported for this agent. However, considering that patients’ safety is the priority, further studies, particularly clinical trials, are suggested to achieve a concrete and comprehensive conclusion.