1. Introduction
In January 2020, a sample collected from Bronchoalveolar lavage fluid of a patient in Wuhan showed the 2019 novel coronavirus (2019-nCoV) and it was confirmed as the cause of a cluster of acute respiratory illnesses in Wuhan, China (1). The virus was named SARS-CoV-2 (severe acute coronavirus 2), and the disease was defined as coronavirus disease 2019 (COVID-19) (2, 3). On February 11, the World Health Organization (WHO) announced COVID-19 as a pandemic. In February 2020, the first infected people were detected in Qom City in Iran, and then the disease spread to other cities over the country. The first pediatric case was reported on January 20, 2020, in a 10-year old boy in China (4). There are limited data on the prevalence of COVID-19 in the pediatric population in China, as children were rarely tested for the virus in the earlier phase of the outbreak, especially in Hubei Province, China, with the highest number of confirmed patients (5).
Coronavirus disease 2019 seems to infect children less than adults, with milder manifestations (6). The common signs and symptoms of COVID19 include fever, cough, rhinorrhea, diarrhea, myalgia, nasal congestion, sore throat, headache, dizziness, nausea, vomiting, abdominal pain, diarrhea, and fatigue, the most common of which are fever and cough. Previously, we had the epidemics of two beta coronaviruses, namely severe acute respiratory syndrome coronavirus (SARS-CoV) (7, 8) and the Middle East respiratory syndrome coronavirus (MERS-CoV) (9, 10), and COVID-19 is the third one. Some studies have shown that patients with SARS-CoV or MERS had liver injuries with elevated liver enzyme and bilirubin levels, which were mainly in mild degrees (10-13). Studies of COVID-19 infection have shown that the incidence of liver injury ranges from 14.8% to 53%, mainly presenting with abnormal alanine transaminase/aspartate aminotransferase (ALT/AST) levels accompanied by slightly elevated serum bilirubin. However, the serum level of alkaline phosphatase (AKP) remains in the normal range in both mild and severe cases (14, 15). The decreased level of albumin can be identified in severe cases ranging between 26.3 and 30.9 g/L. Liver injury was significantly higher in severe COVID-19 patients than in mild cases (16). In the deceased cases of COVID-19, the incidence of liver injury might reach up to 78%. One study reported that serum ALT and AST levels increased up to 7,590 U/L and 1,445 U/L, respectively, in a severe COVID-19 patient (15). According to evidence, the hepatic injury may occur among adult COVID-19 patients. However, there are limited data regarding liver involvement among critical pediatric patients admitted to the PICU.
2. Case Presentation
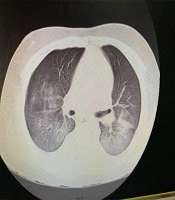
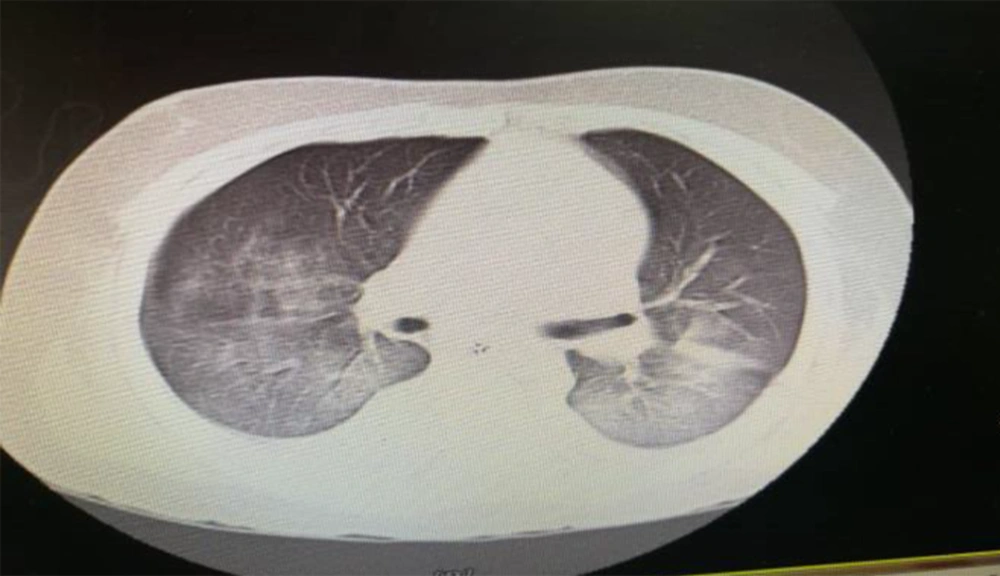
An 11-year- old boy presented with fever and abdominal pain without improvement after an outpatient visit by a family physician. The past medical history was unremarkable. His condition got worse after two days with the increased respiratory rate; thus, he was referred to the hospital and admitted to the pediatric ward. Despite medications during hospitalization, the fever did not subside and he developed yellowish discoloration of the skin and decreased level of consciousness. Therefore, he was transferred to Namazi Hospital, Shiraz, Iran, with the impression of acute liver failure for liver transplantation (after seven days of symptoms onset). His vital signs when arriving in the emergency room were blood pressure 90/45, heart rate 135, respiratory rate 58, and Glasgow Coma scale (GCS) 6/15. In a physical exam, he had weak pulses. He was intubated and transferred to the PICU. An interview with his parents showed that they both had fever and cough with the history of travel to Babol, North of Iran (one of the first COVID-19 epidemic zones in Iran). According to the findings of chest CT scan (bilateral ground glass) (Figure 1), laboratory data, and positive real-time polymerase chain reaction (RT-PCR) of his parents and despite the negative oropharyngeal swab samples of the patient, the routine COVID-19 therapy (hydroxychloroquine plus Kaletra (lopinavir/ritonavir)) started in combination with broad-spectrum antibiotics. It should be noted that the second specimen for COVID-19 was positive. His laboratory data in the PICU are listed in Table 1. Blood culture at PICU admission was negative, and the ceruloplasmin and ammonia levels were in the normal range. However, his condition was deteriorated due to refractory hypotension and decreased urine output. After PICU admission, he suddenly experienced an atrial fibrillation (AF) and asystole without response to cardiopulmonary resuscitation (CPR) and expired.
| Laboratory Variable | Variable Level | Reference Interval |
|---|---|---|
| White blood cells, count/mL | 6900 | 4000 - 11000 |
| Lymphocyte | 690 | - |
| C-reactive protein, mg/L | 150 | < 6 |
| Blood urea nitrogen, mg/L | 100 | 8 - 20 |
| creatinine | 0.1 | - |
| Aspartate transaminase, U/L | 2030 | M: < 37; F: < 31 |
| Alanine aminotransferase, U/L | 690 | F: < 31; M: < 41 |
| Alkaline phosphatase | 387 | - |
| Total bilirubin | 35.4 | 0.1 - 1.2 |
| Direct bilirubin | 21.6 | < 0.3 |
| LKM Ab | 2 | < 18 |
| Lactate dehydrogenase, U/L | 5660 | < 480 |
| Troponin, ng/mL | 3343 | < 19 |
| D.dimer, ng/mL | 10000 | < 500 |
| PaO2-FIO2 ratio | 200 | - |
| Procalcitonin | 10.1 | ≤ 0.3 |
| G6PD | Sufficient | - |
| ESR | 19 | - |
| Partial trombone time | 15.9 | - |
| International normalized ratio (INR) | 1.3 | - |
| Albumin | 3.2 | - |
3. Discussion
Coronavirus disease 2019 is a novel infectious disease caused by SARS-CoV-2. In December 2019, pneumonia cases of unknown origin were first identified in Wuhan, China, and then rapidly spread to the whole country. Up to date, most countries worldwide have been affected. Our case was an 11-year-old boy infected with COVID-19 that presented with acute hepatitis. His EBV/CMV tests were negative and his parents were positive for COVID-19. The patient had a negative test for COVID-19, followed by a positive result. Generally, the mortality rate of COVID-19 in children is very low, and this case is the first presentation of such degree of severity with acute hepatitis. We assessed viral hepatitis serology, hepatic auto-antibodies, 24 h urine, ceruloplasmin, and copper level to rule out other differential diagnoses. Mild cases of COVID-19 show symptoms of fever, fatigue, dry cough, vomiting, and diarrhea. In severe cases, respiratory distress and/or hypoxemia occur one week after the disease onset, leading to acute respiratory distress syndrome (ARDS), septic shock, metabolic acidosis, and even death (14). Liver damage in patients with COVID-19 infection might be directly induced by a viral infection of liver cells. Approximately, the viral RNA is detected in stool and blood samples of 2% - 10% of the patients (17). The current evidence implicates the possibility of viral infection in the liver. This virus binds to the angiotensin-converting enzyme 2 (ACE2) receptor to enter the target cells (18), where the virus replicates and subsequently infects other cells in the upper respiratory tract and lung tissue. Recent studies of COVID-19 have shown that the possibility of liver injury ranged from 14.8% to 53%, mainly indicated by abnormal ALT/AST levels accompanied by slightly elevated bilirubin levels (14, 15, 18-27). Albumin decreases in severe cases with a range of 26.3 - 30.9 g/L (15). In deceased cases of COVID-19, the incidence of liver injury might reach up to 58.06% (27) to 78% (26). Liver impairment may also be due to drug hepatotoxicity, which might explain the large variation observed across different cohorts. In addition, immune-mediated inflammation, such as cytokine storm and pneumonia-associated hypoxia, might contribute to liver injury or even develop into liver failure in critically ill COVID-19 patients. Bangash et al. (28) study of liver injury of COVID-19 indicated that the post-mortem liver biopsy of a COVID-19 patient showed only micro-vesicular steatosis, which is common in patients with sepsis (28). Fan et al. (21) reported that one in five patients had elevated levels of AST or ALT, which was not so high. Based on these data, they suggested that COVID-19-related liver injury is relatively mild. They also indicated that patients who developed liver damage were more likely to have higher inflammatory indices (C-reactive protein and procalcitonin) and mostly had a fever, which could be related to the immune response to the viral infection (21). Some other studies have found that liver damage is more likely to happen in severe cases of pneumonia, which is suspected to be associated with inflammatory cytokines (14). These results suggest that liver injury can occur among COVID-19 patients. So far, there is insufficient evidence that liver failure occurs in COVID-19 patients with chronic liver disease, such as chronic hepatitis B or C.
3.1. Conclusions
According to the results of the present study, it is proposed to consider COVID-19 infection during any onset of acute hepatitis. In addition, it should be noted that almost all of the previous studies have reported the evidence of hepatic damage only among adults with COVID-19 infection. In contrast, the current study revealed credible evidence regarding the susceptibility of children to liver involvement following this infection.