1. Background
Hepatitis B is a viral infection that represents a life-threatening public health issue for more than 250 million people worldwide (1). The prevalence of the hepatitis B virus (HBV) has been reported to range from 0.1% to 20% of populations in different parts of the world (2). It has been predicted that similar prevalence rates will continue for the next 40 - 50 years, and 20 million deaths will occur because of HBV infection between 2015 and 2030 (2, 3). In epidemiological studies in Turkey, hepatitis B surface antigen (HBsAg) positivity was found to be approximately 4%, making Turkey an intermediate endemic region (4, 5).
In the treatment of chronic hepatitis B (CHB), HBsAg loss, which is the optimal endpoint, rarely occurs (6-8). Covalently closed circular (ccc) DNA, which represents the stable form of HBV-DNA and serves as a substrate for all viral mRNA synthesis, has the highest resistance to antiviral therapy and host immune response (3, 9). Even after antiviral treatment or spontaneous HBsAg seroconversion, this molecule cannot be eradicated from hepatocytes. For this reason, long-term antiviral therapy is required to avoid adverse consequences, such as hepatocellular adenocarcinoma (HCC) and cirrhosis (3). Tenofovir, one of the agents used in treatment modalities, is a nucleotide analog that inhibits HBV-DNA polymerase and HIV reverse transcriptase (9). The fact that tenofovir is a highly potent molecule with a high resistance barrier makes it capable of resulting in negative HBV-DNA levels in treatment-compliant patients (3, 8). However, the primary disadvantage of this treatment is that prolonged treatment periods increase the cumulative side effects of the drugs over time (3, 8, 10).
Tenofovir has two formulations for the treatment of HBV infections: tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide fumarate (TAF). Prior studies have reported that the long-term use of TDF results in decreased bone mineral density (BMD) and renal toxicity (11). In comparison to TDF, TAF penetrates the whole hepatocyte without being eliminated due to its longer plasma half-life and greater plasma stability and provides therapeutic effects in much lower doses (12, 13). These properties make it preferable for the long-term treatment of CHB (8). Literature recommends switching patients who are at high risk for the complications that develop from long-term treatment, particularly those related to the bones and kidneys, from TDF to TAF or entecavir (ETV) (3, 14, 15). Also, TAF is recommended for HBV prophylaxis in immunosuppressive patient groups at high risk for bone and kidney side effects (3, 16).
2. Objectives
These data have been obtained through animal experiments and phase studies. Thus, the use of TAF needs to be investigated with real-life data. The current study aimed to report the real-life experience of patients in the South Anatolian region of Turkey within the first six months of switching from TDF to TAF due to the presence or risk of osteoporosis, a deterioration in kidney function, hypophosphatemia, bone density loss, or other adverse events.
3. Methods
This multicenter and retrospective study was conducted by analyzing the data of 14 healthcare centers in Turkey. The group of patients included in the survey was named the Pythagoras Cohort. Pythagoras, known as the “father of numbers”, made significant contributions to philosophy and science with the communities he founded. Believing that numbers are the ultimate facts, we decided to present TAF real-life data with this name. These patients have not been included in another study cohort previously. The local ethics committee approved the study. The initial data, which derived from the endpoint of the treatment for those who were treatment-experienced (especially with TDF), were compared to the data for the third and sixth months of TAF (Vemlidy® 25 mg) treatment. Patients received one dose of TAF daily as recommended in the guideline (3). We obtained patient data from the information system of each hospital. The study only included patients aged 18 years and older who were started on TAF for an appropriate indication as determined by the infectious diseases and gastroenterology clinics in the participating healthcare centers from January to September 2019.
During the initial TAF treatment and follow-ups, information regarding the patients’ gender, body mass index (BMI), pulse (pulse/min), blood pressure (mmHg), antiviral therapy experience, interferon history, hemodialysis status, corticosteroid use, further drug use, non-TDF drug use affecting BMD, and history of chronic diseases, osteoporosis, cirrhosis, organ transplantation, and HIV infection was gathered. Measurements of HBV-DNA were also recorded.
The reasons for starting TAF treatment were classified as naivety, presence of proteinuria, history of drug use affecting BMD, presence of osteoporosis, low phosphorus (< 2.5 mg/dL), chronic steroid use, history of atraumatic bone fracture, estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, dialysis status, and renal transplantation history. The study also examined the BMI (kg/m2), pulse (pulse/min), blood pressure (mmHg), leukocyte counts, alanine aminotransferase (ALT; U/L), aspartate aminotransferase (AST; U/L), phosphorus (mg/dL), cholesterol (mg/dL), LDL (mg/dL), HDL (mg/dL), triglycerides (mg/dL), international normalized ratio (INR), total bilirubin (mg/dL), creatinine (mg/dL), albumin (g/dL), alpha-fetoprotein (AFP; U/L), gamma-glutamyl transferase (GGT; U/L), and eGFR (mL/min/1.73 m2) measurements, in addition to determining the presence of proteinuria in the initial visit and three-month follow-up after TAF treatment. The initial and sixth-month hip and spine T-scores were evaluated for BMD controls. In addition, we examined the pharmacovigilance reports as an evaluation of the side effects of TAF.
3.1. Statistical Analyses
The categorical data were presented as frequency distributions and percentages, and the continuous variables were presented as means (± standard deviations) and medians (minimum and maximum). The chi-square test was used to compare the categorical data. The normal distribution of the continuous variables was evaluated with the Kolmogorov-Smirnov test, and the Mann-Whitney U-test was used to compare non-parametric data for the independent groups. For the dependent groups, the Friedman test and the Wilcoxon signed-rank test were used to compare the recurrent measurements of BMI, pulse, leukocyte counts, ALT, AST, phosphorus, cholesterol, LDL, HDL, triglyceride, INR, total bilirubin, creatine, albumin, AFP, GGT, and eGFR after treatment. Cochran’s Q test and McNemar’s test were used to compare blood pressure and the occurrence of proteinuria.
4. Results
4.1. Baseline Data for the TAF Treatment
The mean age of the patients was 47.40 ± 14.5, and 327 of the participants (68.1%) were male. Of the 480 participants, 78.1% were treatment-experienced (TDF, n = 340; 70.8 %), and 21.9% were treatment-naive. Table 1 presents the demographic characteristics, treatment modalities, underlying diseases, and vital signs of the patients grouped by their antiviral treatment histories.
Drug use that affected BMD (42.9%) and osteoporosis (22.3%) were found to be the most common reasons for a patient starting/switching to TAF treatment. A total of 583 reasons were reported for the initiation of TAF, and 103 patients reported more than one reason. Table 2 presents the distribution of the reasons for initiating TAF treatment.
| Total | Treatment-naive, 105 (21.9) | Treatment-Experience, 375 (78.1) | Pa | |
|---|---|---|---|---|
| Gender (n = 480) | 0.235 | |||
| Male | 327 (68.1) | 68 (64.8) | 259 (69.1) | |
| Female | 153 (31.9) | 37 (35.2) | 116 (30.9) | |
| Age (n = 480) | 46.5 (19 - 89) | 58 (21 - 89) | 44 (19 - 80) | < 0.001 |
| Chronic disease (n = 462)b | 172 (37.2) | 52 (50.0) | 120 (33.5) | 0.002 |
| Osteoporosis (n = 448) | 139 (31.0) | 11 (12.6) | 128 (35.5) | < 0.001 |
| Cirrhosis (n = 569) | 98 (20.9) | 48 (49.5) | 50 (13.4) | < 0.001 |
| Hemodialysis (n = 474) | 12 (2.5) | 5 (4.9) | 7 (1.9) | 0.092 |
| Solid organ transplantation (n = 470) | 97 (20.6) | 48 (49.0) | 49 (13.2) | < 0.001 |
| HIV infection (n = 477) | 0 | 0 | 0 | |
| Duration from the diagnosis of HBV infection (months) (n = 448) (min-max) | 5 (1 - 360) | 5 (1 - 240) | 72 (18 - 360) | < 0.001 |
| Familial history of hepatitis B (n = 480) | 185 (38.5) | 9 (8.6) | 176 (46.9) | < 0.001 |
| Body mass index (kg/m2) (n = 309) (min-max) | 26.6 (16.9 - 50.1) | 23.1 (19.3 - 35.6) | 26.7 (16.9 - 50.2) | 0.028 |
| Pulse (beats/min) (n = 240) (min-max) | 80 (65 - 120) | 80 (65 - 110) | 80 (65 - 120) | 0.234 |
| Blood pressure (mmHg) (n = 345) | 0.073 | |||
| Normal | 312 (90.4) | 34 (81.0) | 278 (91.7) | |
| Hypertensive | 30 (8.7) | 7 (16.7) | 23 (7.6) | |
| Hypotensive | 3 (0.9) | 1 (2.4) | 2 (0.7) | |
| Treatment experience | 375 (78.1) | |||
| TDF | 325 (67.7) | |||
| ETV | 15 (3.1) | |||
| LAM | 8 (1.6) | |||
| LdT | 5 (1.0) | |||
| TDF + LAM | 8 (1.6) | |||
| TDF + ETV | 3 (0.6) | |||
| TDF + LdT | 4 (0.8) | |||
| Unknown | 7 (1.4) | |||
| Total TDF | 340 (70.8) | |||
| Interferon history (n = 480) | 73 (15.2) | 4 (3.8) | 69 (18.4) | < 0.001 |
| Steroid usage (n = 480) | 78 (74.3) | 64 (17.1) | < 0.001 | |
| Additional drug usage (n = 480) | 259 (54.0) | 95 (90.5) | 164 (43.7) | < 0.001 |
| Non-TDF drug use affecting BMD (n = 480) | 299 (62.3) | 88 (83.8) | 211 (56.3) | < 0.001 |
| eGFR (n = 55/236) (mean ± SD) | 91.77 ± 25.07 | 85.93 ± 30.46 | 93.16 ± 23.50 | 0.113 |
| HBV DNA (n = 94/344) (mean ± SD) | 1.6 × 106 ± 19 × 106 | 5 × 106 ± 3 × 106 | 5 × 105 ± 9 × 106 | 0.590 |
| Hip T score (n = 1/79) (mean ± SD) | -1.35 ± 1.27 | -1.2 | -1.46 ± 0.73 | 0.196 |
| Spine T score (n = 1/79) (mean ± SD) | -1.93 ± 1.31 | -2.6 | -1.49 ± 1.04 | 0.992 |
Abbreviations: BMD, bone mineral density; eGFR, estimated glomerular filtration rate; ETV, entecavir; HBV, hepatitis B virus; HIV, human immunodeficiency virus; LAM, lamivudine; LdT, telbivudine; SD, standard deviation; TDF, tenofovir disoproxil fumarate.
aChi-square and Mann-Whitney U-test.
bChronic diseases: chronic heart failure, diabetes mellitus, asthma, chronic obstructive pulmonary disease, malignancy, and rheumatological disease.
| Primary Reason, No. (%) | All Reasons, No. (%) | |
|---|---|---|
| Naive | 87 (18.1) | 105 (18.0) |
| Proteinuria | 18 (3.8) | 20 (3.4) |
| Drug usage affecting BMD | 181 (37.7) | 250 (42.9) |
| Osteoporosis | 128 (26.7) | 130 (22.3) |
| Phosphorus level < 2.5, mg/dL | 31 (6.5) | 35 (6.0) |
| Chronic steroid usage | 3 (0.6) | 4 (0.7) |
| History of nontraumatic bone fracture | 0 (0) | 0 (0) |
| eGFR < 60, mL/min/1.73 m2 | 17 (3.5) | 23 (3.9) |
| Dialysis | 2 (0.4) | 3 (0.5) |
| Renal transplantation | 6 (1.3) | 6 (1.0) |
| Switching to a more potent drug | 7 (1.5) | 7 (1.2) |
zAbbreviations: BMD, bone mineral density; eGFR, estimated glomerular filtration rate; TAF, tenofovir alafenamide fumarate.
4.2. The First Six Months of TAF Treatment
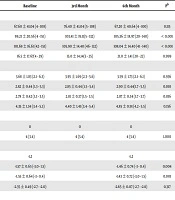
Patients’ vitals and biomarkers were evaluated at the beginning of treatment, at three months, and at six months. The changes are presented in Table 3. A statistically significant decrease was detected in the BMI of patients after the initiation of TAF treatment (P < 0.05) due to the lower values in the third and sixth months (P = 0.001 and P = 0.008). The mean LDL and cholesterol levels increased when compared with those measured at the onset of the treatment; however, this increase was not statistically significant.
| Baseline | 3rd Month | 6th Month | Pa | |
|---|---|---|---|---|
| Body mass index (kg/m2) (n = 119) | 27.1 ± 3.5 (18.7 - 38.8) | 26.8 ± 3.5 (18.2 - 37.9) | 26.8 ± 3.5 (18.2 - 37.9) | 0.002 |
| Pulse (beats/min) (n = 116) | 78.7 ± 9.3 (65 - 110) | 78.4 ± 8.6 (65 - 100) | 78.8 ± 8.3 (67 - 99) | 0.717 |
| Blood pressure (mmHg) (n = 122) | 0.368 | |||
| Normal | 116 (95.0) | 117 (95.9) | 115 (94.2) | |
| Hypertension | 6 (5.0) | 5 (4.1) | 7 (5.8) | |
| Hypotension | 0 | 0 | 0 | |
| Leukocyte count (103/L) (n = 135) | 6817.5 ± 1907.6 (1100 - 14920) | 6874.8 ± 1953.4 (2330 - 18400) | 6724.4 ± 1646.9 (2860 - 13170) | 0.765 |
| ALT (U/L) (n = 134) | 35.5 ± 20.4 (9 - 229) | 35.2 ± 15.8 (5 - 142) | 35.2 ± 18.5 (5 - 183) | 0.815 |
| AST (U/L) (n = 134) | 33.5 ± 16.0 (12 - 175) | 34.4 ± 12.9 (4 - 101) | 35.4 ± 15.4 (12 - 154) | 0.075 |
| INR (n = 123) | 1.2 ± 0.1 (0.9 - 1.7) | 1.1 ± 0.1 (0.8 - 1.5) | 1.1 ± 0.1 (0.8 - 1.4) | 0.387 |
| Cholesterol (mg/dL) (n = 100) | 195.5 ± 22.0 (145 - 235) | 200.3 ± 26.6 (122 - 320) | 203.7 ± 20.7 (156 - 253) | 0.212 |
| LDL (mg/dL) (n = 95) | 102.9 ± 13.6 (84 - 167) | 106.6 ± 13.9 (88 - 167) | 109.7 ± 16.9 (71 - 172) | 0.050 |
| HDL (mg/dL) (n = 104) | 57.8 ± 14.1 (28 - 98) | 59.4 ± 14.8 (34 - 89) | 59.3 ± 18.3 (34 - 89) | 0.318 |
| Triglycerides (mg/dL) (n = 104) | 193.8 ± 33.9 (50 - 243) | 190.9 ± 30.2 (55 - 235) | 194.8 ± 30.9 (70 - 276) | 0.442 |
| Total bilirubin (mg/dL) (n = 112) | 1.1 ± 0.3 (0.3 - 2.2) | 1.1 ± 0.4 (0.3 - 2.2) | 1.1 ± 0.4 (0.3 - 3.2) | 0.212 |
| Creatinine (mg/dL) (n = 128) | 1.1 ± 1.2 (0.3 - 11.2) | 1.0 ± 1.2 (0.4 - 10.1) | 0.9 ± 1.1 (0.4 - 10.1) | 0.026 |
| Albumin (g/dL) (n = 128) | 3.4 ± 0.5 (2.3 - 5.0) | 3.4 ± 0.5 (2.3 - 4.7) | 3.4 ± 0.5 (2.3 - 4.9) | 0.074 |
| GGT (U/L) (n = 91) | 31.0 ± 12.8 (5 -80) | 31.2 ± 12.2 (5 -70) | 32.6 ± 17.5 (8 -144) | 0.587 |
| AFP (U/L) (n = 118) | 2.9 ± 1.1 (1.0 - 10.5) | 2.9 ± 0.9 (0.4 - 7.1) | 2.8 ± 0.9 (0.8 - 6.4) | 0.573 |
| HBV-DNA (IU/mL) treatment-naive (n = 4) | 929 ± 1691 | 0 | 0 | 0.135 |
| HBV-DNA (IU/mL) treatment-experienced (n = 120) | 5135 ± 56232 | 444 ± 3844 | 193 ± 1796 | 0.747 |
Abbreviations: AFP, alfa fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; GGT, gamma-glutamyl transferase; HDL, high-density lipoprotein; INR, international normalized ratio; LDL, low-density lipoprotein.
aFriedman F values are expressed as mean ± SD (min-max).
4.3. Viral Response
Among the patients whose baseline values were recorded (n = 438), 82.2% had an HBV-DNA level below 14 IU/mL at baseline, which increased to 94.7% of patients in the sixth month. This frequency increased from 67.0% to 100% in the naive patients and from 86.3% to 94.4% in those who were treatment-experienced. The initial HBV-DNA levels were below 14 IU/mL in 67.0% of the treatment-naive patients. The reason for this high frequency is that 85 patients (81.0%) (regardless of the HBV-DNA levels) had started TAF due to prophylaxis. However, HBV-DNA levels were evaluated at three time-points for 124 patients, of which 120 (96.8%) were treatment-experienced, and 4 (3.2%) were treatment naive. No significant differences were detected between the HBV-DNA levels in these three measurements (P > 0.05) (Table 3).
4.4. Safety
Table 4 presents all changes in the eGFR, serum phosphorus, urine protein, and BMD measurements according to previous treatment experiences. The mean eGFR and phosphorus levels increased significantly from the baseline to the sixth month of TAF among the treatment-experienced patients (P < 0.001 and P = 0.010, respectively). This difference was attributed to prior TDF experience. The measurements during follow-up visits were also different from each other. Among the 340 TDF-experienced patients, 237 (69.7%) had their BMD measured upon the initial visit, and 76 (22.3%) were re-evaluated for BMD in the sixth month. For this group, the mean hip and spine T-scores after six months were significantly higher than the initial values (P < 0.001 and P = 0.001, respectively). No significant difference was detected between the mean hip and spine T-scores at the baseline and after six months in those with entecavir experience (P = 0.317 and P = 0.317, respectively). A total of 16 naive patients were assessed for BMD upon the initial visit, but only one patient was evaluated in the sixth month. Therefore, no statistical comparison was made.
| Baseline | 3rd Month | 6th Month | Pb | |
|---|---|---|---|---|
| eGFR (mL/min/1.73 m2) (n = 120) | ||||
| Treatment-naive (n = 5) | 67.60 ± 41.04 (4 - 108) | 76.40 ± 41.04 (5 - 108) | 67.20 ± 40.64 (4 - 100) | 0.115 |
| Treatment-experienced (n = 105) | 99.21 ± 20.56 (4 - 151) | 103.41 ± 19.11(5 - 132) | 105.36 ± 18.97 (20 - 140) | < 0.001 |
| TDF-experienced (n = 93) | 101.69 ± 16.61 (42 - 151) | 105.90 ± 14.40 (46 - 132) | 108.04 ± 14.40 (41 - 140) | < 0.001 |
| Entecavir-experienced (n = 2) | 16.5 ± 17.67(4 - 29) | 15.0 ± 14.14(5 - 25) | 21.0 ± 1.41 (20 - 22) | 0.999 |
| Phosphorus (mg/dL) (n = 121) | ||||
| Treatment-naive (n = 4) | 3.68 ± 1.83 (2.1 - 6.3) | 3.95 ± 1.69 (2.3 - 5.6) | 3.59 ± 1.73 (2.1 - 6.1) | 0.936 |
| Treatment-experienced (n = 117) | 2.82 ± 0.44 (1.3 - 5.3) | 2.85 ± 0.44 (1.5 - 5.4) | 2.90 ± 0.44(1.7 - 5.5) | 0.010 |
| TDF-experienced (n = 113) | 2.78 ± 0.42 (1.3 - 3.5) | 2.81 ± 0.37 (1.5 - 3.5) | 2.87 ± 0.34 (1.7 - 3.7) | 0.016 |
| Entecavir-experienced (n = 2) | 4.35 ± 1.34 (3.4 - 5.3) | 4.40 ± 1.41 (3.4 - 5.4) | 4.85 ± 0.91 (4.2 - 5.5) | 0.156 |
| Proteinuria (n = 118) | ||||
| Treatment-naive (n = 2) | 0 | 0 | 0 | |
| Treatment-experienced (n = 116) | 4 (3.4) | 4 (3.4) | 4 (3.4) | 1.000 |
| Hip T-score (n = 79) | ||||
| Treatment-naive (n = 1) | -1.2 | -1.2 | ||
| Treatment-experienced (n = 78) | -1.57 ± 0.65 (-3.0 - 1.5) | -1.46 ± 0.74 (-3 - 0.4) | 0.004 | |
| TDF-experienced (n = 76) | -1.55 ± 0.64 (-3 - 0.4) | -1.43 ± 0.72 (-3.0 - 1.5) | 0.001 | |
| Entecavir-experienced (n = 2) | -2.35 ± 0.49 (-2.7 - -2.0) | -2.65 ± 0.07 (-2.7 - -2.6) | 0.317 | |
| Spine T-score (n = 79) | ||||
| Treatment naive (n = 1) | - 2.6 | - 2.6 | ||
| Treatment experience (n = 78) | -1.77 ± 0.83 (-3.9 - 1.4) | -1.50 ± 1.05 (-3.5 - 2.5) | < 0.001 | |
| TDF-experienced (n = 76) | -1.74 ± 0.82 (-3.9 - 1.4) | -1.47 ± 1.04 (-3.5 - 2.5) | < 0.001 | |
| Entecavir-experienced (n = 2) | -2.70 ± 0.84 (-3.3 - -2.1) | -2.22 ± 1.48 (-3.3 - -1.2) | 0.317 |
Abbreviations: BMD, bone mineral density; eGFR, estimated glomerular filtration rate; TAF, tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate.
aValues are expressed as mean ± SD (min-max).
bFriedman F, Wilcoxon signed rank test and Cochran’s Q test.
4.5. Side Effects
Table 5 presents the observed side effects of the TAF treatment. Hair loss was the most commonly reported side effect at both time-points. In the third month, the treatment plan for a patient with side effects such as increased blood pressure and hyperesthesia was changed. In the sixth month, the treatment plan was changed for male patients experiencing continued hair loss at both follow-ups.
| Side Effect | 3rd Month (N = 263) | 6th Month (N = 140) |
|---|---|---|
| Side effect | 15 (5.7) | 10 (7.1) |
| Hair loss | 4 (26.7) | 5 (50.0) |
| Dizziness | 3 (20.0) | 1 (10.0) |
| Fatigue | 2 (13.3) | 3 (30.0) |
| Hyperesthesia and hypertension | 1 (6.7) | 0 |
| Vomiting | 1 (6.7) | 0 |
| Weakness | 1 (6.7) | 0 |
| Nausea | 1 (6.7) | 0 |
| Itching | 1 (6.7) | 0 |
| Indigestion | 1 (6.7) | 1 (10.0) |
| No side effect | 248 (94.3) | 130 (92.9) |
5. Discussion
Chronic hepatitis B (CHB) has not yet been eradicated, despite the existence of effective oral antiviral treatment options and an effective vaccine, because of the cccDNA component of the virus. Thus, a cure cannot be achieved with the existing treatment modalities. However, viral suppression is critical for preventing complications from the disease, especially cirrhosis and HCC. These patients should receive antiviral treatment for a long time, but prolonged treatment periods can cause side effects. As in the treatment of all chronic diseases, the ideal approach to CHB is an effective and safe treatment plan. One option is TAF, which has been put into use in the last years and has demonstrated efficacy and safety in phase studies and limited clinical trials. Building on this research, the current study demonstrates the effectiveness and safety of TAF in the treatment of CHB with real-life data for the first six months of TAF therapy in both treatment-experienced and naive patients. Of note, the current multicenter study was also conducted with a larger patient cohort than similar studies in the literature (10, 17).
When using a drug that affects BMD and has side effects relating to bone diseases or kidney dysfunction, a switch from TDF to TAF is recommended (3). In our study, the most common reason for switching to TAF was drug use that affected BMD, and the second reason was osteoporosis, which is consistent with the literature (3, 18). In equivalence studies, TAF was found to be as effective as TDF, which has a high barrier for resistance (19, 20). In a real-life study, the average HBV-DNA levels in the TDF and TAF arms of naive patients were the same at the 48-week follow-up, whereas the antiviral effect at 24 weeks was maintained in those who changed from TDF to TAF (17). In our treatment-experienced patients, we also observed that the antiviral efficacy was maintained.
Renal toxicity is a well-known side effect of TDF treatment. Tenofovir undergoes active renal secretion through organic anion carriers (OAT1 and OAT3), increasing the exposure of the proximal renal tubules to tenofovir (11, 21-23). However, TAF is not a substrate for renal OATs and does not show OAT-induced cytotoxicity (22). Typically, after terminating TDF treatment, tubular cytotoxicity is eliminated, phosphorus excretion is decreased, and serum phosphorus levels increase (10). In our study, a gradual increase in the serum phosphorus levels of patients was observed during the follow-up period, demonstrating that tubular dysfunction returned after the transition from TDF to TAF. The adverse renal effects of TDF were supported by the fact that decreases in eGFR were relatively lower in those using TAF (19, 20). An increase in eGFR and a decrease in urinary β2-microglobulin/creatinine at an early stage after switching to TAF demonstrated the recovery of the loss of kidney function due to the TDF treatment (17). Similarly, in our study, the mean eGFR values increased significantly in the three- and six-month follow-ups.
The negative effect of TDF on BMD has been associated with proximal renal tubulopathy (PRT) related to phosphorus excretion and increased bone turnover (11). In particular, TDF usage was found to be a risk factor for osteoporotic fracture through an examination of risk factors for BMD in HIV-infected patients (24). For healthy people not infected with HIV, using TDF as pre-exposure prophylaxis caused a significant decrease in BMD when compared with their BMD before the prophylaxis period and the BMD of a placebo group (25). For patients with CHB, previous studies found TDF to be a risk factor for having a T-score of ≤ (-) 1 and a 2% decrease in hip and spine bone density values in the 24th week (15, 26). However, the existing literature has shown significant improvements in bone density for both CHB and HIV-infected patients after switching to TAF (10, 18, 27). In the current study, a significant improvement in bone density was also detected in the sixth month after transitioning to TAF. These results support switching to TAF treatment due to the negative effects of TDF, particularly for BMD.
Although its mechanism is not yet known, TDF therapy has been reported to have a lipid-lowering effect in HIV-infected patients (28). However, its clinical importance is controversial, since it does not cause changes in total or HDL cholesterol. Of note, LDL cholesterol levels were also found to be high (≥ 190 mg/dL) in 6% of the TAF-receiving CHB patients when compared to 1% of the TDF-receiving patients in the 96th week of treatment (29). In addition, a significant increase in LDL cholesterol was observed in HIV-infected patients after switching from TDF to TAF treatment regimens (30, 31). In the current study, the mean total and LDL cholesterol values increased gradually but not significantly during the follow-up process, and the BMI values decreased significantly in the first six months, which is contrary to the values reported in the literature. In general, it is advisable to exercise caution when evaluating the effects, such as weight gain and lipid changes, after especially one year. Therefore, we considered a six-month follow-up to be too early to assess the side effects (31, 32).
Previously published studies have reported the most common side effects associated with TAF treatment to be nausea, vomiting, coughs, headaches, and mild-to-moderate fatigue (13, 33). After treatment with TAF-containing regimens, hair loss was reported in a case series of female patients infected with HIV (34). In the present study, treatment was terminated when this side effect was experienced by a male patient, although hair loss is more likely to impact the quality of life and social functioning of women. For this reason, before treatment, patients must be informed of this side effect, which is known to be reversible before reaching a more severe level.
There were some limitations to the current study. First, the number of naive patients who received TAF treatment was low. Second, the current study was conducted with data based on six months of observations; therefore, a long-term evaluation of safety and antiviral activity was not possible. Third, the limited number of HBeAg(+) patients also prevented more reliable results, especially in terms of viral response. Finally, the lack of data on all biomarkers for renal function and bone turnover can be considered a limitation.
5.1. Conclusions
After switching to TAF treatment, patients who had previous treatment experience demonstrated sustained antiviral efficacy. In addition, TAF replacement reversed the side effects of TDF treatment on the kidneys and bones. In our study, the increased eGFR and bone density observed under TAF treatment supported the view that TAF treatment offered less systemic exposure and a higher safety margin than TDF treatment. Although there was no difference in the initial analysis of the total and LDL cholesterol after transitioning to TAF treatment, these results should be assessed in future studies. In light of the real-life data and the early results of our research, TAF seems to be an effective choice for CHB patients who need long-term treatment, especially when there are concerns about the kidney and bone-related side effects of TDF treatment. Although the six-month data on the Pythagoras Cohort were promising, further studies are needed to evaluate these data over the long term.