1. Background
Much evidence indicate that OBI (presence of HBV DNA in the absence of HBsAg) may occur in any clinical setting (1). More attention is paying to this health problem, as it may affect the outcome of HBV infection. OBI may contribute to acute exacerbation and development of HBV-associated diseases such as progression of liver disease, cirrhosis, and hepatocellular carcinoma (HCC) (2). Reactivation of HBV infection may occur at the stage of OBI and is now a well-recognized complication in patients who have received cytotoxic or immunosuppressive therapy, leading to severe or even fulminant hepatic failure sometimes indistinguishable from de novo acute infection (3, 4). There have been several reports in the past few years on the reactivation of HBV in patients with rheumatic disorders following the administration of immunosuppressive drugs with an incidence rate of 1.1- 17.2% (5, 6). As the majority of patients in those studies were HBsAg positive, Centers for Disease Control
(CDC) and the American College of Rheumatology recommended screening for HBV serology before biologic and immunosuppressive therapy (7, 8).
Behcet's disease (BD) is a chronic multisystem vasculitis with an uncertain etiology. Several factors have been suggested as triggers in the etiopathogenesis of BD, including viral agents (9). However, an association between viral agents and BD has not been confirmed yet (9). On the other hand, immunosuppression caused by drugs used by BD patients makes them prone to the threat of HBV reactivation regardless of having overt or occult infections. According to the best knowledge of the authors, there is no report on the prevalence of OBI in patients with BD.
2. Objectives
Hence, the current study aimed to, firstly, assess the prevalence of overt and OBI in a series of patients with BD, and, secondly, looking for the potential association between the clinical and therapeutic status of these patients and the occurrence of OBI.
3. Methods
3.1. Patient's Selection
Among patients with BD referred to the Behcet's disease clinic in Rheumatology Research Center (RRC) at Shariati hospital during 2015-2016, those with the following inclusion criteria were consecutively selected: older than 16 years old with a definitive diagnosis of BD according to the International Criteria for Behcet's Disease (ICBD) (10). Those with other rheumatic diseases were excluded. Clinical files of all patients were reviewed to collect data on demographic characteristics (age, sex, and disease duration), different clinical manifestations, pathology test, HLA typing for B51, and medications used.
The study protocol was approved by the Ethical Committee of the Tehran University of Medical Sciences (TUMS). All experiments were performed in compliance with relevant laws and institutional guidelines and in accordance with the ethical standards of the Declaration of Helsinki and its amendments. Informed consent was obtained from all patients.
3.2. Serology, DNA Extraction, and Polymerase Chain Reaction
Ten ml aliquots of whole blood samples were withdrawn from each participant, and the serum was separated. HBV serological markers, including HBsAg and HBeAg/anti-HBe, were examined by enzyme-linked immunosorbent assay (ELISA) kits manufactured by Diapro (Milan, Italy) in the Hepatitis B laboratory at TUMS. HBV DNA was extracted from a 200 µl of sera using Qiagen Mini Blood Kit (Qiagen, Hilden, Germany) with a procedure adapted from the manufacturer's instructions. DNA was eluted using 100 µl of elution buffer, stored in -20°C.
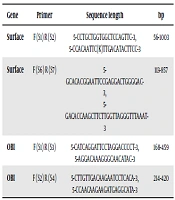
For the identification of OBI, three types of polymerase chain reactions (PCRs) were performed. HBV DNA was initially determined in all samples by real-time PCR (Fast-Track Diagnostics, Luxembourg). Then, all positive samples, regardless of their viral load levels, were selected for conventional PCR reactions using two different approaches (Table 1). For the identification of HBV DNA, two pairs of primers were used based on the highly conserved region of HBsAg as another screening methodology proposed by the Taormina expert meeting on OBI (11). Finally, the positive OBI samples were analyzed for mutation using HBsAg nested PCR (12).
| Gene | Primer | Sequence length | bp |
|---|---|---|---|
| Surface | F (S1) R (S2) | 5-CCTGCTGGTGGCTCCAGTTC-3, 5-CCACAATTC(K)TTGACATACTTCC-3 | 56-1003 |
| Surface | F (S6) R (S7) | 5-GCACACGGAATTCCGAGGACTGGGGAC-3, 5-GACACCAAGCTTCTTGGTTAGGGTTTAAAT-3 | 113-857 |
| OBI | F (S1) R (S3) | 5-CATCAGGATTCCTAGGACCCCT-3, 5-AGGACAAAGGGCAACATAC-3 | 168-459 |
| OBI | F (S2) R (S4) | 5-CTTGTTGACAAGAATCCTCACA-3, 5-CCAACAAGAAGATGAGGCATA-3 | 214-420 |
3.3. Direct Sequencing and Mutational Analysis
Direct sequencing of surface genes was carried out (Perkin Elmer ABI-3130XL DNA Sequencer, Foster city, CA, USA) using 0.5 μl of appropriate primers S6 and S7 for the surface gene (Table 1). The results were analyzed using Chromas (version 2.1.1.) and BioEdit (version 7.0.5.3.) software. The HBsAg genotype/subtype of the sequences was defined by substitutions in the 'a' determinant between codons 122 and 160 inclusive. With regards to the adequate number of Iranian taxa deposited in GenBank and the National Center for Biotechnology Information (NCBI), for sequencing alignment comparison, any amino acid difference between isolates was defined as “mutation”.
3.4. Statistical Analysis
Statistical analysis was performed using IBM SPSS version 20 (IBM Corp., Armonk, NY, USA). Data were reported as the mean ± standard deviation (SD). The comparisons between the groups were done using either the Chi-square test or Fisher's exact test. For all comparisons, statistical significance was considered when P value < 0.05.
4. Results
Of 1500 patients with BD attending the Behcet's disease clinic during the study period, 220 were eligible for inclusion. The mean age of BD patients was 39.24 ± 10.57. One-hundred-thirty-four (62.9%) were male, and the mean disease duration was 14.13 ± 8.63 years. No HBsAg positive case was found; however, HBV DNA was found in 19/220 (8.6%) patients assigned as being positive for OBI. The median viral load value was 475.84 copy/mL. Two patients (1%) were positive for anti-HBc; both were positive for OBI.
Only clinical files of 166 patients (10 positives for OBI) were complete, and their data (demographic, different clinical manifestations, pathology tests, HLA typing, and medications history) were accessible for further analysis. The data of BD patients who were OBI positive (group I) were compared with OBI negative patients (group II). There was no difference between the two groups concerning the demographic characteristics, including age, sex, or disease duration. We found no correlation between OBI positivity and different major or minor clinical manifestations. OBI-positive patients showed a higher rate of positive pathergy test (70% vs. 38.4% with an odds ratio of 3.71); however, the difference was not statistically significant (P = 0.092). It seems that HLA-B51 genetic background has no effect on the susceptibility to OBI in BD patients (Table 2).
| Characteristics | OBI positive (N = 10) | OBI negative (N = 156) | P value | OR (95% CI) |
|---|---|---|---|---|
| Age (Year)a | 36.66 ± 12.30 | 38.58 ± 9.86 | 0.58 | NA |
| Disease Duration (years)a | 14.50 ± 7.10 | 14.10 ± 8.73 | 0.89 | NA |
| Maleb | 7/10 (70) | 100/156 (62.2) | 0.99 | 1.307 (0.325-5.25) |
| Oral ulceb | 10/10 (100) | 156/156 (100) | NA | NA |
| Genital ulcerb | 7/10 (70) | 94/156 (60.2) | 0.742 | 1.54 (0.383-6.179) |
| Skinb | 5/10 (50) | 91/156 (58.3) | 0.744 | 0.71 (0.20-2.57) |
| Folliculitisb | 5/10 (50) | 72/156 (46.2) | 0.99 | 1.153 (0.321-4.143) |
| Uveitisb | 5/10 (50) | 94/156 (60.2) | 0.527 | 0.66 (0.183-2.372) |
| Retinalb | 5/10 (50) | 91/156 (58.3) | 0.744 | 0.71 (0.20-2.57) |
| Jointb | 5/10 (50) | 91/156 (58.3) | 0.744 | 0.71 (0.20-2.57) |
| Vascularb | 0 | 14/156 (9) | NA | NA |
| Neurologicalb | 1 (10) | 14/156 (9) | 0.913 | 1.13 (0.133-9.52) |
| Gastrointestinalb | 0 | 9/156 (5.8) | NA | NA |
| Positive pathergyb | 7/10 (70) | 60/156 (38.4) | 0.092 | 3.71 (0.92-14.85) |
| Positive HLA-B51b | 2/6 (33.33) | 48/106 (45.28) | 0.69 | 0.604 (0.106-3.42) |
| Immunomodulatory drugsb | 9/10 (90) | 136/156 (87.2) | 0.99 | 1.324 (0.159-11.01) |
| Cytotoxic drugsb | 8/10 (80) | 125/156 (80.1) | 0.99 | 0.99 (0.201-4.907) |
| Corticosteroid drugsb | 9/10 (90) | 152/156 (97.44) | 0.270 | 0.237 (0.024-2.344) |
Abbreviation: NA, Not applicable.
aindicated as mean ± standard deviation.
bindicated as No. (%).
Concerning the medications used, no substantial correlation was found between any types of medications (immunomodulatory, cytotoxic, or corticosteroids) and the occurrence of OBI (Table 2). Upon direct sequencing of the whole surface gene for PCR positive- OBI cases, no mutation was found within or outside of “a” determinant region (results not shown).
5. Discussion
Albeit the pathogenesis of BD is unknown, both genetic and environmental factors leading to various immunological changes have been proposed as underlying triggers (9). A possible association between BD and some viruses have been suggested, but none of those viruses have been isolated for reproduction (9, 13). Among different viral agents, hepatitis C virus, cytomegalovirus (CMV), Epstein-Barr virus, parvovirus B19, varicella-zoster virus, and herpes simplex virus-1 (HSV-1) had more evidence for association with BD (14). Hepatitis viruses have been recommended as causative agents for BD due to the incidence of vasculitis in both conditions (9, 13). In the previous few reports on the association of BD and HBV, the probable association has been detected mainly by HBV serology (9, 13, 15-17) (Table 3). The reported prevalence of HBsAg and anti-HBc in BD patients were 2.9 % and 17.7%, respectively. Three (out of 5) available studies are performed in Turkey, an area known for high BD prevalence. Only Akaogi et al. (15) tested HBV DNA in HBV positive patients (8 out of 68, 11.8%,) without directly referring to OBI terminology. Iran has been considered to be a country with a low prevalence of HBsAg. Based on Hajarizadeh's meta-analysis in 2017, the prevalence of HBsAg and anti-HBc Ab among the Iranian general population is estimated at 1.79% and 13.59%, respectively (18). While this study showed that 8.6% of Iranian BD patients were infected with the cryptic form of HBV infection, this rate still is far from the current situation for HBsAg positivity or overt infection in the nation, which deserves further attention as a special group.
| Author/Year | Country | Sample size | OBI | HBV-DNA No (%) | HBsAg (%) | Anti-HBs | Anti-HBc |
|---|---|---|---|---|---|---|---|
| Akaogi/2000 (15) | Japan | 68 | NA | 8 (11.8) | 2 (2.9) | 11 (16.2) | 12 (17.7) |
| Aksu/1999 (9) | Turkey | 124 | NA | NA | 5 (4) | 39 (31) | NA |
| Sebnam/2005 (13) | Turkey | 35 | NA | NA | 1 (2.9) | 16 (45.7) | 11 (31.4) |
| Etem/2013 (16) | Turkey | 56 | NA | NA | 2 (3.57) | 9 (16) | NA |
| Farajzadeh/2005 (17) | Iran | 48 | NA | NA | 1 (2.1) | NA | NA |
| Present study | Iran | 220 | 19 (8.6) | 19 (8.6) | 0 (0) | NA | 2 (0.9) |
Abbreviation: NA, Not applicable.
Regarding the causal association between BD and OBI, previous studies could not reach a definite conclusion. The main objective of the present study was only to show the prevalence of OBI in BD, and not to find such causative association between them. However, due to this relatively high prevalence of OBI among BD subjects, a possible association, either causative or only as an association, might be suggested. Considering the small sample size of positive cases in the present study, further investigations (not only serologic but also by molecular HBV evaluation) are needed to clarify this potential association.
The mean viral load between all OBI-positive patients was below 500 copy/mL, which is consistent with other previously published data, as, in a majority of OBI patients, the level of DNA is too low to be detected by current molecular assays. Also, the level of HBsAg usually might be under the detection limit of current serological tests. To the best of our knowledge, this survey is the first published data on the prevalence of OBI among BD patients.
Reactivation of OBI following immunosuppressive therapy is a well-known complication in rheumatic diseases. HBV reactivation defines by a sudden rise in HBV replication often associated with clinical signs of hepatocellular injury. The main cause of such reactivation has been attributed to immunosuppression caused by DMARDs, both biologic and conventional, higher among the former group (20%- 50% versus 14% to 72%, respectively) (19, 20). Corticosteroids could be associated with OBI reactivation due to the existence of a glucocorticoid response element within the HBV genome. Regarding the potential threat of HBV reactivation in OBI-infected patients, a meta-analysis carried out on rheumatic conditions, showed that 15.4% of HBV reactivation among overt HBV were carriers compared to 1-5% in those who were infected by OBI (20). Despite BD patients in our study did not present any sign of HBV reactivation during their follow-up, we believe that OBI endangers patients for reactivation and developing an overt feature of HBV infection in the future.
It is necessary to mention some limitations and biases of our study. First, the cross-sectional study design is not ideal for identifying risk factors or clinical relevance of biological findings. Second, the relatively small number of cases may have caused the lack of statistical significance for some potential associations.
In conclusion, the relatively high prevalence of OBI in our BD patients cannot be ignored. It reminds the importance of checking HBV infection in BD patients at least prior to start immunosuppressive medications. This study may suggest the need for future studies regarding the association between HBV (either overt or occult infection) and BD patients.