1. Background
Excessive daytime sleepiness stemming from nighttime sleep disturbances is prevalent among individuals diagnosed with chronic hepatitis (1, 2). In Saudi Arabia, the prevalence of excessive daytime sleepiness was higher in patients with hepatitis C and induced liver cirrhosis than in healthy subjects (1). A case-control study observed that more than 50% of patients with chronic hepatitis C and mild liver disease complained about chronic fatigue, daytime sleepiness, and poor sleep quality (2). Furthermore, sleep disturbance in patients with hepatitis B liver cirrhosis has also been reported (3).
The precise pathophysiologic mechanisms underlying sleep disturbance in patients with liver cirrhosis remain to be investigated (4). In addition to the dysfunction of neural circuits responsible for controlling wakefulness and sleep states, the involvement of upper airway edema and disrupted circadian rhythms is also suggested (1). Comorbid conditions may also predispose patients to sleep disturbances or excessive daytime sleepiness; for example, patients with gastroesophageal reflux disease (GERD) who are troubled by sleep disturbances often self-report daytime sleepiness and are found to have decreased quality of life (4).
Although patients with hepatitis B and C are reported to have difficulty sleeping, either falling asleep or staying asleep (1-3), relevant studies on daytime sleepiness in hepatitis are limited. To gain an overview of associations between daytime sleepiness and hepatitis in Taiwan, we conducted the present prospective study.
2. Objectives
This prospective pilot study aimed to evaluate the association between daytime sleepiness and chronic hepatitis as well as identifying risk and protective factors associated with daytime sleepiness in patients with hepatitis B and/or hepatitis C but without treatment of hepatitis.
3. Methods
3.1. Study Design and Population
This prospective cross-sectional questionnaire-based pilot study aimed to evaluate the association between daytime sleepiness and chronic hepatitis. Consecutive outpatients with fatigue who visited the Department of Gastroenterology and Hepatology at our hospital from August 2016 to July 2017 were screened for eligibility. Inclusion criteria were adults aged 20 years and older, with laboratory and imaging results suggestive of early chronic hepatitis and/or liver disease, and without antiviral treatment. Only patients with minor liver inflammation (early stage of hepatitis) who had not yet been treated for hepatitis were included. Liver inflammation was determined based on ultrasonic examination and serum levels of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT). Patients with a history or evidence of liver cancer or liver transplantation were excluded.
At each patient’s first visit, clinical laboratory tests were performed to measure serum GOT, GPT, and lipid levels, and screening for hepatitis B and C. Liver cirrhosis was diagnosed based on parenchymal liver disease score derived from ultrasound imaging as previously described (5). Based on the test results, these outpatients were first classified into two groups of with and without hepatitis. They were further reclassified into one of the five following subgroups: hepatitis B (HBV positive), hepatitis C (HCV positive), hepatitis B + C, other types of hepatitis, and no hepatitis (controls). At 3-month follow-up visits, patients who agreed to participate in this study and signed the informed consent were enrolled and were asked to complete the questionnaires at the hospital.
3.2. Ethical Considerations
The study protocol was reviewed and approved by the Internal Review Board of our hospital (code: 201600332B0). All included patients provided signed informed consent to participate.
3.3. Main Measures
Two Chinese versions of questionnaires were used to evaluate the gastroesophageal reflux (defined as GERDQ score ≥ 12) and daytime sleepiness (defined as ESS score ≥ 8), as described below.
3.3.1. Gastroesophageal Reflux Disease Questionnaire
The Chinese version of the gastroesophageal Reflux Disease questionnaire (GERDQ) was used to collect self-reported data about daytime sleepiness. The reliability of this Chinese version of the questionnaire was previously validated, and the GERDQ score ≥ 12 was defined as daytime sleepiness (6).
3.3.2. Epworth Sleepiness Scale
The Chinese version of the Epworth Sleepiness scale (ESS) was used to collect self-reported data about daytime sleepiness. The reliability of this questionnaire was validated previously, and the ESS score ≥ 8 was defined as daytime sleepiness (7).
3.4. Study Variables
Information about snoring, hypnotic use, and comorbidities was obtained retrospectively from patients’ medical records. All included patients did not have severe chronic liver diseases or psychiatric disorders, and they did not take hypnotics on a regular basis. Although some of them had minor sleep problems, they took hypnotics no more than once a week. Therefore, patients with hypnotic use were included for a larger sample size.
3.5. Statistical Analysis
Continuous variables are presented as mean and standard deviation (mean ± SD); categorical variables are presented as frequency and percentage. Associations between hepatitis and ESS score/daytime sleepiness were evaluated using linear and logistic regression models. Subgroup analysis was conducted to identify the risk factors for daytime sleepiness in patients with hepatitis B or hepatitis C. Associations between risk factors and daytime sleepiness were evaluated using univariate logistic regression analysis. The significance level was set as two-sided P < 0.05. All statistical analyses were performed using the R version 3.6.2 statistical software.
4. Results
In total 131 patients with fatigue, who were screened among consecutive patients visiting the Department of Gastroenterology and Hepatology at the study hospital, were included in this study. All patients did not have a history of receiving any treatment for hepatitis. Of them, 42 were diagnosed with hepatitis B, 62 with hepatitis C, 9 had both hepatitis B and hepatitis C, and 4 had other types of hepatitis (2 with alcoholic hepatitis and 2 with non-alcoholic fatty liver disease). In addition, 14 participants, who were not diagnosed with any type of hepatitis, served as the control group.
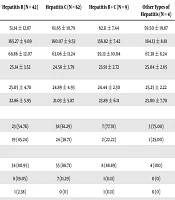
The distribution of participants’ baseline demographic and clinical characteristics are provided in Table 1. The mean age of the total population was 57.9 years; mean body mass index (BMI) was 24.9 kg/m2; mean GOT was 25.0 U/L; and mean GPT was 22.1 U/L. Most participants did not have snoring problems (hepatitis B, 54.76%; hepatitis C, 61.29%; B + C, 77.78%; other types, 75%) and did not use hypnotics (hepatitis B, 80.95%; hepatitis C, 88.71%; B + C, 88.89%; other types, 100%). Only 2 (out of 117) hepatitis patients had liver cirrhosis, indicating that most included hepatitis patients were at the early stage of disease. The most common comorbidity in the total population was hypertension (n = 13), followed by diabetes mellitus (DM, n = 8), chronic obstructive pulmonary disease (COPD, n = 3), and chronic kidney disease (CKD, n = 1). In the total population, 38 (29.01%) participants had gastroesophageal reflux (with a GERDQ score ≥ 12), and 13 (9.92%) had daytime sleepiness (with an ESS score ≥ 8). In total, 11.90% of patients had daytime sleepiness, while 80.6% of those diagnosed with hepatitis B and hepatitis C were suffering from this problem. No patient in the control group presented daytime sleepiness (Table 1).
| Overall (N = 131) | Control (N = 14) | Hepatitis B (N = 42) | Hepatitis C (N = 62) | Hepatitis B + C (N = 9) | Other Types of Hepatitis (N = 4) | |
|---|---|---|---|---|---|---|
| Age, y | 57.94 ± 12.57 | 58.79 ± 12.59 | 51.14 ± 12.87 | 61.65 ± 10.79 | 62.11 ± 7.44 | 59.50 ± 19.87 |
| Body height, cm | 161.76 ± 9.65 | 161.21 ± 9.20 | 165.27 ± 9.69 | 160.07 ± 9.53 | 156.82 ± 7.42 | 164.13 ± 8.81 |
| Body weight, kg | 65.65 ± 12.80 | 71.19 ± 19.48 | 68.86 ± 12.07 | 63.06 ± 11.24 | 59.13 ± 10.04 | 67.38 ± 8.24 |
| Body mass index, kg/m2 | 24.99 ± 3.80 | 27.00 ± 5.09 | 25.14 ± 3.52 | 24.58 ± 3.79 | 23.91 ± 2.72 | 25.04 ± 2.65 |
| GOT, U/L | 25.04 ± 4.68 | 26.00 ± 5.42 | 25.05 ± 4.70 | 24.89 ± 4.93 | 24.44 ± 2.30 | 25.25 ± 2.22 |
| GPT, U/L | 22.14 ± 6.07 | 23.50 ± 6.85 | 22.86 ± 5.95 | 21.03 ± 5.87 | 23.89 ± 6.11 | 23.00 ± 7.70 |
| Snoring | ||||||
| No | 77 (58.78) | 6 (42.86) | 23 (54.76) | 38 (61.29) | 7 (77.78) | 3 (75.00) |
| Yes | 54 (41.22) | 8 (57.14) | 19 (45.24) | 24 (38.71) | 2 (22.22) | 1 (25.00) |
| Hypnotic use | ||||||
| No | 113 (86.26) | 12 (14.29) | 34 (80.95) | 55 (88.71) | 8 (88.89) | 4 (100) |
| Yes | 18 (13.74) | 2 (85.71) | 8 (19.05) | 7 (11.29) | 1 (11.11) | 0 (0) |
| Cirrhosis | 2 (1.53) | 0 (0) | 1 (2.38) | 0 (0) | 1 (11.11) | 0 (0) |
| Comorbidity | ||||||
| COPD | 3 (2.29) | 0 (0) | 2 (4.76) | 0 (0) | 0 (0) | 1 (25.00) |
| CKD | 1 (0.76) | 0 (0) | 1 (2.38) | 0 (0) | 0 (0) | 0 (0) |
| DM | 8 (6.11) | 0 (0) | 1 (2.38) | 6 (9.68) | 1(11.11) | 0 (0) |
| HTN | 13 (9.92) | 1 (7.14) | 4 (9.52) | 8 (12.90) | 0 (0) | 0 (0) |
| GERDQ | 10.49 ± 4.36 | 10.07 ± 4.12 | 10.33 ± 3.97 | 10.50 ± 4.15 | 11.89 ± 7.80 | 10.25 ± 3.77 |
| < 12 | 93 (70.99) | 10 (71.43) | 29 (69.05) | 46 (74.19) | 6 (66.67) | 2 (50) |
| ≥ 12 | 38 (29.01) | 4 (28.57) | 13 (30.95) | 16 (25.81) | 3 (33.33) | 2 (50) |
| ESS | 3.49 ± 2.86 | 2.71 ± 1.82 | 3.05 ± 2.81 | 3.71 ± 2.94 | 5.22 ± 3.83 | 3.50 ± 1.29 |
| < 8 | 118 (90.08) | 14 (100) | 37 (88.10) | 57 (91.94) | 6 (66.67) | 4 (100) |
| ≥ 8 | 13 (9.92) | 0 (0) | 5 (11.90) | 5 (8.06) | 3 (33.33) | 0 (0) |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ESS, Epworth Sleepiness scale; GERDQ, gastroesophageal reflux disease questionnaire; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; HTN, hypertension.
aValues are expressed as mean ± SD or No. (%).
Table 2 shows the results of linear and logistic regression analyses for the association between daytime sleepiness and hepatitis in all patients, regardless of etiology. In the linear regression model, the ESS score was positively associated with hepatitis, but it was not statistically significant. Moreover, we could not use logistic regression analysis since no patient in the reference (control) group had daytime sleepiness (Table 2).
| ESS Score, Β ± SE | Daytime Sleepiness, OR (95% CI) | |
|---|---|---|
| Control | Reference | Reference |
| Hepatitis | 0.87 ± 0.81 | NA |
Abbreviations: β, beta coefficient; ESS, Epworth Sleepiness Scale; SE, standard error.
Table 3 shows the risk, and protective factors for daytime sleepiness in patients with hepatitis B or hepatitis C. Patients with hepatitis B + C or other types of hepatitis were not subjected to subgroup analysis because the number of these patients was limited. Univariate regression analysis revealed that among hepatitis B patients, those with gastroesophageal reflux had significantly higher odds of daytime sleepiness [odds ratio (OR) = 12.44, 95% confidence interval (CI) = 1.59 - 261.02], compared to those without gastroesophageal reflux. In contrast, gastroesophageal reflux was not significantly associated with daytime sleepiness in patients with hepatitis C (Table 3). However, hypertension was associated with significantly increased odds of daytime sleepiness in patients with hepatitis C (OR = 15.6, 95% CI = 2.13 - 143.21), but not in hepatitis B patients. Higher body height and elevated serum GOT levels were associated with significantly lower odds of daytime sleepiness (body height: OR = 0.81, 95% CI = 0.65 - 0.93; GOT: OR = 0.76, 95% CI = 0.55 - 0.95) in patients with hepatitis C (Table 3).
| Hepatitis B, OR (95% CI) | Hepatitis C, OR (95% CI) | |
|---|---|---|
| Age, y | 1.02 (0.95 - 1.11) | 1.09 (0.99 - 1.25) |
| Body height, cm | 0.96 (0.88 - 1.06) | 0.81 (0.65 - 0.93)a |
| Body weight, kg | 0.94 (0.86 - 1.02) | 0.95 (0.86 - 1.03) |
| Body mass index, kg/m2 | 0.83 (0.57 - 1.11) | 1.13 (0.09 - 1.42) |
| GOT, U/L | 0.89 (0.70 - 1.09) | 0.76 (0.55 - 0.95)a |
| GPT, U/L | 0.88 (0.71 - 1.05) | 0.95 (0.79 - 1.12) |
| Snoring | ||
| No | Reference | Reference |
| Yes | 0.78 (0.09 - 5.27) | 1.06 (0.13 - 6.89) |
| Hypnotic use | ||
| No | Reference | Reference |
| Yes | NA | NA |
| COPD | ||
| No | Reference | Reference |
| Yes | 8.90 (0.32 - 260.91) | NA |
| CKD | ||
| No | Reference | Reference |
| Yes | NA | NA |
| DM | ||
| No | Reference | Reference |
| Yes | NA | 2.60 (0.12 - 22.57) |
| Hypertension | ||
| No | Reference | Reference |
| Yes | 2.83 (0.12 - 29.49) | 15.6 (2.13 - 143.21)a |
| GERDQ | 1.24 (0.99 - 1.59) | 1.13 (0.93 - 1.35) |
| < 12 | Reference | Reference |
| ≥ 12 | 12.44 (1.59 - 261.02) a | 2.05 (0.25 - 13.61) |
Abbreviations: CI, confidence interval; GERDQ, gastroesophageal reflux disease questionnaire; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; OR, odds ratio.
aSignificant (P < 0.05).
5. Discussion
In the present prospective pilot study, the majority of patients were early-stage hepatitis patients and had no history of receiving treatment. Besides, only 2 (out of 117) patients had liver cirrhosis. In all subgroups, most patients reported having sleep problems, mainly snoring. The most common comorbidity in the total population was hypertension, followed by diabetes. Nearly one-third of all patients had gastroesophageal reflux. Among patients with hepatitis B, those with gastroesophageal reflux had significantly higher odds of daytime sleepiness, while those with hepatitis C did not. In hepatitis C patients, hypertension was associated with significantly increased odds of daytime sleepiness, but not in hepatitis B patients. Concerning the protective factors, taller body height and elevated serum GOT levels were significantly associated with lower odds of daytime sleepiness in hepatitis C patients, but not in hepatitis B patients.
In the hepatitis B and hepatitis C groups, daytime sleepiness was more common in hepatitis B (11.9%) patients than in those with hepatitis C (8.06%). However, when testing all hepatitis patients for daytime sleepiness using the ESS, no statistically significant difference was observed between the scores of hepatitis patients and controls. It worth noting that the ESS instrument has been questioned for its commonly used cutoff point of 11, which has been suggested to be insufficient for clinical practice (8).
Hepatitis C patients with cirrhosis, with a history of short sleep duration, had a higher prevalence of excessive daytime sleepiness (1). Among all extrahepatic manifestations of hepatitis, the central nervous system is more often affected (9), and chronic fatigue is reported in more than half of hepatitis C patients, even without advanced disease or while receiving antiviral therapy (2). Patients with chronic hepatitis B who have progressed to liver cirrhosis have higher levels of sleep disturbances, daytime fatigue, mood disturbances, and attention/memory deficits (3, 10). As such, besides the integral factors of hepatitis itself, psychological factors may be a cause of sleep disturbances, including disruption of melatonin levels that may be associated with depression and anxiety. Fluctuations of viral load and antiviral therapy may also contribute to sleep disturbances and the resulting daytime sleepiness (10). In hepatitis B patients, both inactive carriers and those undergoing antiviral treatment exhibited psychological symptoms such as anxiety and hostility as well as sleep disturbances, which all are correlated with poorer health-related quality of life (11). In a large multi-center cohort study of hepatitis C patients, pain, fatigue, and sleep disturbances were common and frequently severe (12). Our non-significant results linking hepatitis B and C with daytime sleepiness can possibly be explained by the high prevalence of early-stage hepatitis in the study population, and also that they only reported “snoring” and not more severe sleep disturbances such as sleep apnea, which is positively associated with daytime sleepiness (1). However, snoring is a feature of obstructive sleep apnea (OSA) and is still reported to be a risk factor for daytime sleepiness (13). More studies are needed to determine the symptoms that may be resolved by eradicating the virus.
Our patient population only included two patients with liver cirrhosis, so we yielded no valid results for cirrhosis patients. GOT and GPT values in the non-hepatitis control group were actually comparable to those of patients with hepatitis, suggesting that controls and patients with hepatitis had a similar liver function and confirming that most included hepatitis patients were at an early stage of the disease. However, it has been demonstrated that biochemical markers of liver function (in primary liver cirrhosis) are not necessarily correlated with sleep problems and related daytime sleepiness (14). Nevertheless, studies that specifically evaluated daytime sleepiness in cirrhosis patients found that excessive daytime sleepiness was especially high in hepatitis-associated cirrhosis patients (1, 2). Likewise, sleep quality was impaired, and the risk of OSA was increased in patients with compensated liver cirrhosis, and as the severity of liver cirrhosis increased, the sleep patterns and excess daytime sleepiness increased (15).
In the present prospective pilot study, using subgroup analysis, a series of certain risk and/or protective factors associated with daytime sleepiness in patients with hepatitis B or hepatitis C were identified; however, there was an obvious difference between the two groups concerning these factors: gastroesophageal reflux was a risk factor in hepatitis B patients exclusively, while hypertension was an exclusive risk factor in hepatitis C patients. In a previous study, nocturnal gastroesophageal reflux is reported as a risk factor for daytime sleepiness in women and is mentioned to have a more negative effect when combined with snoring (13). Also, snoring and gastrointestinal reflux are lifestyle factors that are both associated with obesity, but the association with daytime sleepiness is independent of obesity, indicating that obesity is not a risk factor for daytime sleepiness (16).
A study on hepatitis C patients with underlying comorbidities reported no correlation between scores of tests for health and mental status (cirrhosis-related symptom score [CSS]; and short-form [SF-36] health survey) (17). However, we found no comparable results of other studies consistent with our findings concerning possible protective factors of taller body height and elevated GOT in hepatitis C patients, except that laboratory values for liver parameters (GPT and GOT) were not associated with daytime sleepiness and altered sleep patterns in patients with compensated liver cirrhosis (15). More studies are needed to confirm these protective factors as well as identifying other factors.
In contrast to the previous studies that explored the effects of anti-viral treatment on sleeping problems in hepatitis patients (17-19), the present study included early-stage hepatitis patients with no history of receiving treatment, which precluded evaluating the possible impacts of medication use.
Severe sleep problems were also absent in hepatitis patients in the present pilot study. However, in a comparison of the severity of sleep problems between patients with hepatitis C who received interferon vs. interferon-free medications, the interferon therapies caused significant sleep problems compared to the interferon-free therapies (19). Understandably, those authors suggested that the nature of sleep problems in hepatitis patients was not well understood, and cautioned that inadequate sleep may lead to exhaustion, irritability, and mood swings, exacerbating overall health status, and quality of life while trying to rid the body of the virus (19). Raison et al. (20) demonstrated that chronic exposure to immune cytokines in chronic hepatitis patients treated with interferon-alpha was associated with reduced continuity and depth of sleep and resulted in insomnia and hyperarousal. Hence, immune cytokines may be a link between chronic inflammatory conditions, altered evening cortisol production, motor slowing, and daytime fatigue. Furthermore, the importance of differentiating the effects of treatment vs. the symptom burden of hepatitis, as well as the further elucidation of causal pathways, have been suggested (12).
The present prospective pilot study has several limitations. First, we only included outpatients who were newly diagnosed with chronic hepatitis but had not yet received any treatment, which allowed us to evaluate hepatitis-associated sleepiness without the possible influence of antiviral medications; however, due to this special inclusion criterion, the sample size of this pilot study is relatively small. In addition, a few patients, who used hypnotics no more than once a week, were included in this study for a larger sample size. But, to rule out the possibility of confounding effects, such patients may be excluded in the future large-scale multi-center study. The Chinese version of the GERDQ do not contain items on nocturnal GERD, so whether the included patients have nocturnal GERD is unclear. Furthermore, the present research is a cross-sectional study conducted in a single medical center, which might include biases. Hence, caution should be taken when generalizing results to other contexts or population groups. Although patients with hepatitis B + C had the highest percentage of daytime sleepiness, but due to the small sample size (n = 9), such high daytime sleepiness in patients with hepatitis B + C needs to be confirmed by studies with larger populations. This was a prospective pilot study with only early-stage hepatitis patients (only two had cirrhosis), and therefore, further studies are needed to explore this issue. Future prospective studies should involve a larger multi-center sample that includes patients with hepatitis B and hepatitis C at different stages, including those with and without hepatitis-associated cirrhosis.
5.1. Conclusions
This study demonstrated that gastroesophageal reflux is a risk factor for daytime sleepiness in patients diagnosed with hepatitis B, but not hepatitis C. Hypertension was found to be associated with significantly increased odds of daytime sleepiness in patients with hepatitis C, but not in hepatitis B. Also, taller body height and elevated serum GOT levels are protective factors for daytime sleepiness in patients with hepatitis C.