1. Background
Hepatitis C is a major health problem in the world. As known, HCV infection is usually asymptomatic, and HCV infected people are usually not clinically aware of their infection until their liver problem symptoms are detected. Globally, 70 - 80% of infected individuals experience a chronic infection with a 10-30% risk of cirrhosis and liver carcinoma (1, 2). Thus, HCV as a transfusion-transmitted virus is among significant issues of blood safety, and HCV transmission via transfusion is among the primary concerns of Blood Transfusion (BT) systems (1). Multi-layered strategies such as high-quality donor selection and sensitive blood testing are used to increase blood, blood components, and safety of plasma derivatives (3). However, donors whose last donation has had a positive result in HCV screening but a negative result in previous donations or donors with a history of risk behaviors expose recipients of previous donations to an increased risk of acquired HCV infection. The situation occurs when a person donates in the early phase of HCV infection, during which the HCV antibody could not be detected in the HCV screening test (the window period), even with the implementation of more sensitive molecular screening tests. A subsequent donation of such a donor is detected as repeatedly reactive in the screening test. As a result, all their previous collections could be at increased risk of HCV transmission (4).
Experiences from the look-back process in different countries showed the successful distinguishing of infected recipients not aware of their infection (5). The HCV look-back process aims at improving blood safety and the general health of society via the identification of donation with an increased risk of HCV transmission (4). The World Health Organization (WHO) in “recommendations for the production, control, and regulation of human plasma for fractionation” recommends that in blood establishments, the look-back procedure should be performed to exclude donation from processing and inform fractionators about the situation. The WHO also recommends donor notification and counseling for donor health and blood safety (4). With the harsher application of the recommendations, blood establishments have faced a growing discard rate of blood products and increasing notifications about blood banks in hospitals, as well as about the risk of HCV infection.
In Iran, the contract fractionation of plasma has been implemented by the Iranian Blood Transfusion Organization (IBTO) since 2004, followed by the look-back process (6). According to the IBTO Standard Operating Procedures (SOPs), a blood donor with a repeatedly reactive result in HCV screening and a negative result in a previous donation is subjected to the look-back process. In the case of a repeatedly positive HCV donor with a history of blood donation(s), the look-back process is initiated retrospectively to detect the date of the last negative donation of the donor during five years. In the next step, six months before the last negative donation, all blood and blood products collected from the donor are excluded from further processing and discarded (obviously if they have not been used). In addition, if the stored plasma product is not shipped, it will be discarded immediately according to the IBTO SOPs. If the stored plasma product is already shipped, IBTO will notify the fractionator based on their agreement. However, data is not available in Iran about the look-back investigation of recipients of blood and blood products from a previous negative donation to a consequent positive one.
2. Objectives
The present study aimed at determining possible HCV transmission in recipients of blood components collected from HCV confirmed blood donors using molecular methods.
3. Methods
3.1. Participants
The study was conducted on HCV-confirmed blood donors across the country during 2015 - 2017. The HCV-confirmed blood donors whose previous donation had positive results in the HCV screening test and accepted to participate in the study were included. The HCV look-back process was performed using a computer database. The subjects were selected according to the IBTO SOPs (described in the introduction section).
3.2. Sample Collection
The selected donors were asked to give blood samples for molecular testing. Blood samples were collected in vacutainer tubes with a gel separator, immediately centrifuged at 3,000 rpm for 10 min, and put in storage at -70 °C until further processing.
3.3. Risk Factor Interview
The subjects were interviewed about demographic characteristics including gender, age, education level, and marital status, as well as common HCV risk factors among Iranian blood donors, including intravenous drug abuse, religious self-flagellation, non-injecting drug abuse, history of blood, transfusion, imprisonment, sharing a personal razor, tattooing, extramarital sexual activity, cupping in an outpatient setting, tooth extraction, surgery, and intramuscular injection by trained physicians (7).
3.4. Look-Back Investigation of Recipients
All the blood components from the previous donations of the subjects were traced. In the case of transfusion of blood components, a treating physician was informed about the possibility of HCV infection via BT. The treating physician evaluated medical records and, if necessary, took a blood sample from a particular recipient and send it to the IBTO centers to test HCV antibodies using assays currently used in IBTO centers to confirm HCV infection using a molecular assay, if necessary.
3.5. Molecular Testing
3.5.1. RNA Extraction
The viral RNA was extracted using the TriPure isolation reagent (Roche, Germany) according to the manufacturer’s instruction. The extracted RNA was eluted in 20 µl elution buffer and stored at -70°C until further processing.
3.5.2. HCV RNA Amplification
The HCV RNA was detected using an in-house one-step TaqMan real-time RT-PCR assay with the LightCycler instrument (Roche, Germany) to amplify a segment of a Non-Coding Region (NCR) of the HCV genome, as described elsewhere (8). The sensitivity of the assay was 15 IU/ml, with a linear range of 101 IU/µl to 104 IU/µl (8). In subjects with undetectable HCV RNA, the donors were asked for the second-time blood sample for repeating HCV RNA testing due to the natural fluctuation of the hepatitis C viral load (9, 10).
3.6. Ethics Statement
The Ethics Committee of High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, was responsible for approving the study (Code No. IR.TMI.REC.1394.1800).
4. Results
4.1. Donors
During the study period, 280 participants were evaluated as potential participants, of whom 276 were first-time donors, nine were repeated donors, and three were regular donors. The first-time donors were excluded from the study because they had no blood donation history. Moreover, the nine repeated donors were not able to be included in the look-back study because of the time interval between their previous negative donations and their consequent positive donations, which was more than five years (data not shown). All the three regular donors were male with the age under 40 years.
The HCV RNA was detected in Donor 3 but was not detected in the related previous donation recipient. Of the two remaining donors with undetectable HCV RNA who were asked to give samples for repeated HCV RNA testing for the second time, Donor 2 did not show HCV RNA. However, Donor 1 did not attend the BT centers, and thus no blood sample was available for HCV RNA retesting. The baseline characteristics and laboratory features of the three regular donors are shown in Table 1.
| Donor Number | Gender | Age (y) | Marital Status | Education Level | HCV RNA |
|---|---|---|---|---|---|
| 1 | Male | 22 | Single | Diploma | Undetectable |
| 2 | Male | 35 | Married | Diploma | Undetectable |
| 3 | Male | 39 | Married | Under diploma | Positive/ 7160 IU/ml |
The time interval between the previous HCV negative donation and the repeatedly positive donation was 11 months and two days, 10 months and 23 days, and nine months and 29 days in Donor 1, Donor 2, and Donor 3, respectively, as shown in Table 2. None of the regular donors donated for six months before the last negative donation.
| Donor Number | Date of Index Donation | Date of Last HCV-Negative Donation | Date of Donation Before Last HCV-Negative Donation | Date of Sampling |
|---|---|---|---|---|
| 1 | 2015.08.12 | 2014.09.10 | 2013.10.121 | 2015.12.08 |
| 2 | 2015.12.13 | 2015.01.20 | 2013.02.28 | 2016.02.16 |
| 3 | 2016.03.16 | 2015.05.17 | 2014.06.29 | 2016.06.22 |
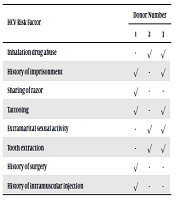
All the regular blood donors reported more than two risk factors. Moreover, Intravenous Drug Abuse (IVD) was not reported by the subjects (Table 3).
| HCV Risk Factor | Donor Number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Inhalation drug abuse | - | √ | √ |
| History of imprisonment | √ | - | √ |
| Sharing of razor | √ | - | - |
| Tattooing | √ | - | √ |
| Extramarital sexual activity | - | √ | √ |
| Tooth extraction | - | √ | √ |
| History of surgery | √ | - | - |
| History of intramuscular injection | √ | - | - |
4.2. Recipients
From the last negative donation of the three regular donors, FFP and RBC units were produced. The FFP unit produced from the last negative donation of Donor 1 was discarded due to a reason other than the results of HCV screening. The RBC unit was delivered to the hospital and transfused to the patient. Making contact with the hospital, no record of recipients hospitalized before 2015 was available, and thus we had no further information.
Given that the HCV RNA was not detected in the plasma unit collected from the last donation of Donor 2, the donor was less likely to be infected with HCV.
The previous FFP unit produced from the negative donation of Donor 3 with positive results in HCV RNA testing was wasted because of a reason not related to the results of HCV screening. The RBC unit was transfused to a woman. The treating physician was informed about the transmission possibility of HCV infection via transfusion. The recipient was recalled, and a sample of her blood was taken, as requested. The results of the HCV antibody and HCV RNA testing were negative and undetectable, respectively. The reason might be that the recipient probably indicated the absence of the HCV antibody and HCV RNA. The approximate time interval between receiving the RBC unit and testing the HCV antibody was four months.
5. Discussion
In this study, HCV infection transmission through BT was investigated. During the study period, seroconversion and the HCV antibody were confirmed in all the three regular donors. The approximate time interval between the previous donations of the donors and their index donations was 9 - 11 months, which is more than six months considered as the window period of HCV infection (1). Due to the long interval between transfers of units to hospitals, hospitals received notifications about the risk of HCV transmission after RBC unit transfusion.
The results of this look-back study showed that there was no evidence of HCV transmission resulting in the HCV infection of recipients via BT. Moreover, HCV RNA was not detected in Donor 1 and Donor 2. Due to natural fluctuations of the hepatitis C viral load, a repeat of the qualitative PCR assay on a new sample was considered (9, 10). Despite the repeated notification of Donor 1, he refused to give a second blood sample at BT centers for HCV RNA retesting. A low response rate of seropositive blood donors to notifications has been reported in some studies (11, 12). Although HCV RNA was detected in Donor 3, it was not detected in the recipient.
The sequence analysis of HCV strains has been introduced as a powerful epidemiological tool for tracing transmission routes (13). This study aimed to perform the HCV sequencing of a segment in the non-structural region of the HCV genome, as well as HCV genotyping (14) if the regular donors and recipients of their blood components had detectable HCV RNA. However, HCV RNA was presented in none of the donor-recipient pairs.
In the present study, although none of the regular donors reported IVD as the most common HCV risk factor among Iranian blood donors, they reported at least three of the commonest HCV risk factors among Iranian blood donors (7). According to IBTO instructions, potential donors reporting the IVD risk factor are permanently rejected from the donation, while potential donors reporting other risk factors are only not allowed to donate temporarily. Considering that all the HCV risk factors were integrated into the pre-donation questionnaire, highlighting some risk factors reported by the regular blood donors was a remarkable finding. Regular donors are regarded as the safest blood donors due to recurring donations; they are aware of the donor selection process, and their health status is checked regularly. However, the findings showed that such donors also knew that they would be temporarily rejected from donation if they disclosed any risk factors during the pre-donation interview. On the other hand, some physicians may do not care much about the pre-donation questionnaire, supposing that regular donors are safe.
Some limitations should be noted in this study. First, as it was the first study on HCV look back based on tracing back recipients, we included HCV antibody confirmed (and not all repeatedly reactive) blood donors in the study. Second, not all HCV seropositive confirmed blood donors were willing to participate in the study, which affected the sample size. Third, hospital medical records were not available for all recipients. A successful look-back process needs document recording of all procedures from veins of blood donors to those of recipients.
In conclusion, in this small-scale look-back study, evidence of HCV infection transfusion through BT was not detected. Due to the critical role of the look-back procedure in ensuring and increasing blood safety, tracing back recipients along with performing the look-back process for plasma fractionation, which is routinely performed in IBTO, is recommended. In addition, paying more attention to the blood donor selection process, even for regular donors, is highly recommended as it results in improving blood safety. Finally, an accurate record-keeping system in hospitals is recommended for a successful look-back process.