1. Background
Non-alcoholic fatty liver disease (NAFLD) has emerged as an epidemic in developed countries, mostly affecting obese and diabetic individuals (1). In NAFLD, there are three types of liver changes: Steatosis (fat accumulation), steatohepatitis, and fibrosis (2). The rate of NAFLD progression is higher in women than in men (3). Generally, exercise is an important component of lifestyle modification to manage NAFLD. Also, combinations of aerobic and resistance training, targeting the mediators of adipose tissue (adipokines), have prominent effects on NAFLD (1).
There is an association between NAFLD and insulin resistance, as 70 - 80% of obese and diabetic individuals suffer from NAFLD (4). Insulin resistance and obesity result in NAFLD by affecting the formation of adipokines and triglyceride synthesis, increasing lipolysis, and simultaneously delivering free fatty acids (FFAs) to the liver (5). As a potent anti-inflammatory adipokine, C1q/tumor necrosis factor-related protein 3 (CTRP3) plays a role in regulating lipid metabolism and inhibiting gluconeogenesis; it may also compensate for adiponectin deficiency. The serum CTRP3 level seems to be lower in patients with NAFLD. In a previous study, after a three-year follow-up, the CTRP3 concentration decreased from baseline to follow-up in patients with NAFLD (6). In the literature, the low serum level of CTRP3 has been suggested as a strong predictor of NAFLD among patients recently diagnosed with type 2 diabetes mellitus (7).
So far, very few investigations have examined the effect of exercise training on CTRP3. In a previous study, a three-month combined training program significantly reduced the CTRP3 level in obese women (8). Conversely, another study showed that aerobic training did not change the CTRP3 level (9). Because the baseline CTRP3 concentration varies in different patients (6, 9-11), it can be concluded that the response of CTRP3 to exercise training varies in different populations and may depend on the type and intensity of training.
Few studies have evaluated the effects of different training intensities on NAFLD improvement. According to these studies, the intensity factor is more crucial than the duration or total volume of exercise (12, 13). Moreover, several human and animal studies have shown that interval training reduces the intrahepatic fat content (12, 14, 15). However, a comparison of the effects of exercise intensity on insulin resistance in NAFLD patients has been rarely conducted (12, 16). Physical activity has a dose-effect relationship with fatty liver disease (17). Therefore, the exercise intensity factor can be considered as an important aspect of exercise training, and interventional investigations are needed to confirm the effects of various intensities of exercise training on patients with fatty liver disease (13).
Since insulin resistance is the major cause of fatty liver disease, and studies focusing on the effects of long-term training on CTRP3 in NAFLD patients have not been reviewed so far, it seems necessary to evaluate the effects of different intensities of combined training on this indicator in NAFLD patients.
2. Objectives
This study was conducted to investigate and compare the effects of two types of combined training with two different intensities on the CTRP3 level and insulin resistance in patients with NAFLD.
3. Methods
3.1. Participants
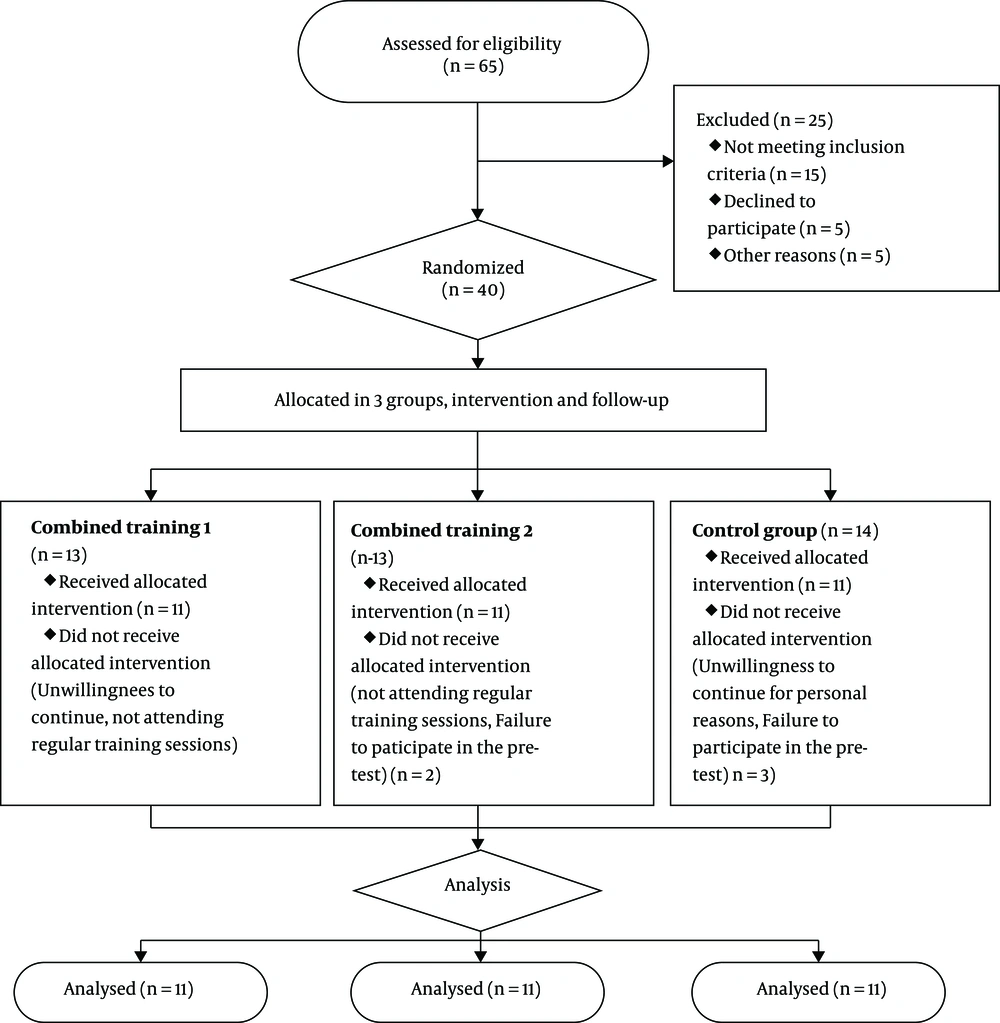
In this semi-experimental study, the recall was applied in the clinics, hospitals, and ultrasound centers of Shahrud, Iran. The inclusion criteria were women with hepatic steatosis, confirmed by ultrasonography, and a hepatic triglyceride content above 5%. On the other hand, the exclusion criteria were as follows: Self-declared patients with a history of heart and kidney disease, viral hepatitis, or other liver diseases, alcohol consumption of more than 20 g/day, uncontrolled hypertension, autoimmune disorders, medical conditions preventing exercise, weight loss > 3 kg in the last three months, regular exercise in the last six months, and more than four sessions of absence in training. As detailed in Figure 1, considering the possible sample attrition, the final analysis was performed on 33 women with NAFLD (mean age: 43.45 ± 7.57 years), selected via available purposeful sampling.
The subjects were divided into three equal groups: experimental group 1, experimental group 2, and control group (n = 11 per group). Patients with low-grade steatosis (grade 1) were randomly divided into groups (simple random selection of subjects based on their numbers). Also, patients with higher grades of steatosis (grade > 1) were divided into groups after matching in terms of drug use and grade of steatosis (simple random selection of subjects based on their numbers). Patients with low-grade steatosis (grade < 2) and some patients with higher grades of steatosis did not take any medications. The patients taking metformin and atorvastatin in both groups received the same drug doses in consultation with a physician.
The Ethics Committee of Hakim Sabzevari University (ID: IR.HSU.REC.1397.004, 2018) approved the present study. Before training in the gym, the subjects completed a consent form and the Physical Activity Readiness Questionnaire (PAR-Q) after they were given a full explanation about the details of the study and the positive effects of regular training on fatty liver disease; they were also familiarized with the training exercises (resistance training [RT] and running on a treadmill) (18). The cost of ultrasound, blood sampling, tests, and the gym was paid for by the researcher. Also, for safety during training, all participants were insured for free for one year.
Both experimental groups participated in three sessions of training per week, in addition to their daily activities. First, RT was performed, followed by an interval training program (aerobic interval training [AIT] or high-intensity interval training [HIIT]) for 12 weeks under the supervision of a sports physiologist. Each training session continued for about 55 - 75 minutes (ten minutes of warm-up, 20 - 30 minutes of RT, 16 - 30 minutes of interval training, and five minutes of cooldown). Since we evaluated a training intervention versus non-training (control group), it was not possible to blind the participants. However, to prevent bias, the laboratory experts were unaware of the subjects’ groups (experimental and control groups) for blood sampling, sample analysis, and statistical analysis.
3.2. RT Program
The RT program consisted of six movements in 2 - 4 circles with 60 - 75% of 1 repetition maximum (1RM) and 8 - 12 repetitions. To apply the overload, the weight increased every two weeks. We considered resting of 30 - 45 seconds between movements and resting in circles of 60 - 90 seconds. The 1RM was calculated using The Brzycki formula:
3.3. AIT Program
The AIT program consisted of four-minute intervals of treadmill running at 70 - 75% of maximum heart rate (HRmax) and two minutes of active rest at 50 - 60% of HRmax. The training started with two four-minute intervals. By adding one interval every three weeks, the training volume reached five intervals of four minutes in the last three weeks. In the first six weeks, the intensity of training was 70% of HRmax, and in the second six weeks, it reached 75% of HRmax. The intensity of interval training was monitored using a Beurer PM62 heart rate monitor (Germany). The HRmax was estimated based on the following formula:
3.4. HIIT Program
The HIIT program consisted of one-minute intervals of treadmill running at 85 - 95% of HRmax and one minute of active rest at 60 - 70% of HRmax. The training started with eight intervals in the first week. By adding one interval every two weeks, the training volume reached 13 intervals of one minute. The exercise intensity also started with 85 - 90% of HRmax in the first six weeks, which reached 90 - 95% of HRmax in the last six weeks.
3.5. Control Group
The control group participated in no exercise training program during the 12-week period of the study. However, the subjects continued their daily physical activities.
3.6. Measurement of Variables
One week before the program, the subjects visited the sonography center to determine the grade of steatosis after eight hours of fasting (LOGIQ P6 Ultrasound General Electric Co., USA). In the pretest phase, blood samples were taken by laboratory specialists from the brachial vein (5 cc) after 12 hours of fasting and 24 hours of inactivity. The plasma concentration of CTRP3 was determined using a specific laboratory kit (Hazngzhou Eastbiopharm Co., China) with a sensitivity of 0.09 ng/mL. Also, the insulin level was measured using a specific kit (Biotech, USA) with a sensitivity of 0.11 µU/mL, based on an ELISA assay. The blood glucose level was measured based on an enzymatic colorimetric method and glucose oxidase technology, using a specific glucose kit (Pars Azmon, Iran). The homeostatic model assessment-insulin resistance (HOMA-IR) index was determined according to the following formula:
The patients’ anthropometric and body composition indices, including height and weight, were measured with light clothing, without shoes, using a body scale (Parisa model, Dena Tozin Co., Iran). Also, the body mass index (BMI) was determined by dividing weight (kg) by the square of height (m2). After 12 weeks, all measurements were repeated, and blood samples were taken again.
3.7. Statistical Analysis
Sampling was performed using G Power software and repeated measures ANOVA at an alpha error level of 0.02. Repeated measures ANOVA was performed to test the study hypothesis. Shapiro-Wilk test was also used for evaluating the normality of errors. Moreover, Tukey's post-hoc test was applied for determining significant differences. SPSS version 24 and R software were used for data analysis at a significant level of 0.05.
4. Results
The mean and standard deviation (SD) of age, weight, BMI, fasting glucose, fasting insulin, and HOMA-IR of subjects in different groups are listed in Table 1. Regarding the baseline values of BMI, insulin and fasting glucose, and CTRP3, there was no significant difference between the two training groups; however, there was a significant difference between the training groups and the control group (P < 0.001).
| Variables | Control Group | Group 1 (RT + AIT) | Group 2 (RT + HIIT) | RM-ANOVA | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | Pre-test | Post-test | F, G × T | P, G × T | |
| Age (y) | 43.82 ± 7.53 | - | 44.45 ± 6.47 | - | 42.09 ± 9.04 | - | - | - |
| Weight (kg) | 77.04 ± 20.53 | 77.76 ± 20.28 | 85.8 ± 15.89 | 86 ± 16.68 | 76.7 ± 10.66 | 76.43 ± 11.41 | 1.24 | 0.25 |
| BMI (kg/m2) | 32.03 ± 7.61 | 31.41 ± 6.74 | 35.71 ± 5.48 | 35.78 ± 5.59 | 30.77 ± 3.53 | 30.37 ± 3.71 | 0.45 | 0.64 |
| CTRP3 (ng/mL) | 10.54 ± 9.74 | 8.54 ± 8.28 | 8.37 ± 2.72 | 10.79 ± 9.87 | 7.76 ± 2.51 | 6.36 ± 1.43 | 5.34 | 0.01a |
| Post hoc (Tukey), P-value | 0.99 | 0.01b | 0.99 | - | ||||
| Fasting glucose (mg/dL) | 89.82 ± 10.94 | 79.91 ± 6.44 | 98.73 ± 21.8 | 81.36 ± 13.22 | 93.91 ± 14.92 | 84.45 ± 15.09 | 5.8 | 0.007 a |
| Post hoc (Tukey), P-value | 0.73 | < 0.001b | 0.08 | - | ||||
| Fasting insulin, (µU/mL) | 11.70 ± 5.46 | 11.49 ± 5.70 | 17.80 ± 8.93 | 14.66 ± 5.43 | 18.34 ± 11.25 | 16.50 ± 9.95 | 4.23 | 0.02 a |
| Post hoc (Tukey), P-value | 0.98 | 0.01b | 0.47 | - | ||||
| HOMA.IR | 2.62 ± 1.35 | 2.24 ± 1.06 | 4.27 ± 2 | 2.89 ± 0.92 | 4.53 ± 3.55 | 3.62 ± 2.79 | 1.8 | 0.182 |
Changes in Body Composition and Biochemical Parameters After a 12-Week Training Intervention in Different Groups
The results showed that the interaction of time × group was significant in terms of CTRP3, fasting insulin, and glucose levels (Table 1).
Regarding the CTRP3 level, the RT + AIT group showed a significant increase compared with the control group (28% increase vs. 19% decrease) (P = 0.01) and the RT + HIIT group (28% increase vs. 18% decrease) (P < 0.001).
Also, the RT + AIT group showed a significant decrease in the fasting insulin and glucose levels compared with the control group (P < 0.001 and P = 0.01, respectively); however, there were no significant differences between the two experimental groups in terms of fasting insulin and glucose levels (P > 0.05). Also, the HOMA-IR index decreased in both group 1 and group 2; however, the difference was not significant compared with the control group (P > 0.05).
5. Discussion
Our results indicated that 12 weeks of combined AIT (with low intensity) and RT training significantly increased the CTRP3 level. Also, a lower intensity of combined training resulted in a greater increase in the CTRP3 level compared with a higher intensity of combined training (28% increase vs. 18% decrease). In this regard, Choi et al. (2013) demonstrated that three months of combined training (aerobic training at 60 - 75% of HRmax and RT) significantly reduced the CTRP3 level in obese women (8). Also, the results reported by Azali-Alamdari et al. (2016) showed that aerobic training in men with metabolic syndrome (60 - 70% of heart rate reserve), despite some enhancements in insulin resistance, had no significant effects on the serum CTRP3 level (9). This finding is inconsistent with the results of group 1 and consistent with the results of group 2 in the present study, probably due to differences in the statistical populations and methods. Patients with NAFLD have lower CTRP3 levels (6). HIIT increases inflammation, whereas moderate-intensity training reduces inflammation (19). Therefore, it can be concluded that in the present study, the combined training program in group 1, compared with the combined training program in group 2, was associated with inflammation as a consequence of NAFLD progression, with a greater increase in the level of CTRP3, and subsequently, the improved inflammatory status of NAFLD.
In the present study, insulin resistance decreased in both experimental groups (a 32% decrease in group 1 and a 20% decrease in group 2); however, the difference was not significant. There was also a significant decrease in the fasting insulin and glucose levels in group 1. Overall, improvement of insulin resistance is one of the mechanisms involved in the effectiveness of exercise training in improving NAFLD (14). It should be noted that the fasting glucose level was normal in all groups, which justifies the non-significant change in group 2. Mechanistically, insulin resistance in the adipose tissue leads to incomplete lipase suppression, resulting in increased lipolysis and release of FFAs in the serum of NAFLD patients (taken up by the liver). Therefore, improved insulin resistance reduces the FFA flux to the liver (20). Long-term training can increase insulin sensitivity by increasing the levels of glucose transporter 4 (GLUT4) and insulin receptor substrates, as well as the muscle mass (> 75% of glucose uptake due to insulin stimulation) (21).
Several metabolic alterations due to exercise training are associated with AMP-activated protein kinase (AMPK) modulation. The AMPK activation affects the liver, heart, and muscles, leading to weight loss and improvement of lipid profile and insulin resistance. Also, high-intensity training results in AMPK activation. On the other hand, a decrease in liver glucose uptake may be associated with a reduction in hepatic lipogenesis. Moreover, AMPK can modulate carbohydrate metabolism by suppressing gluconeogenesis and glycogenesis (22).
Based on the present findings, it can be concluded that 12 weeks of combined training (group 1), by increasing the CTRP3 level and AMPK activation, inhibited gluconeogenesis, decreased the hepatic glucose uptake, and improved fatty liver disease. However, one of the limitations of the present study was that the patients had different grades of steatosis, and the researcher did not have access to samples of similar steatosis grades.
5.1. Conclusion
Based on the findings, combined training (RT + AIT) increased the level of CTRP3 as an anti-inflammatory adipokine and decreased the levels of fasting insulin and glucose, independent of weight loss. This type of combined training, by increasing AMPK activation, improving insulin resistance, and reducing inflammation, probably contributes to the suppression of NAFLD. Therefore, combined training can possibly be used as a non-pharmacological adjunct therapy for the treatment of women with NAFLD and the prevention of the associated inflammation.