1. Background
COVID-19, which originated in the city of Wuhan (China), soon turned into a pandemic, as announced by the World Health Organization on March 11, 2020 (1). This disease is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which is a single-stranded positive-sense RNA virus of the Coronaviridae family (2). In most cases, the disease causes mild symptoms. However, in some cases, especially in the elderly and people with underlying diseases, such as cardiovascular diseases, diabetes, and respiratory diseases, it causes more severe symptoms and even death (3-5).
Because of its high infectivity and the fact that many patients are asymptomatic, the disease quickly became a pandemic (6). The average incubation period of COVID-19 is 5.1 days, and each person, on average, can infect 2 to 3 other people (7). Although respiratory symptoms and pulmonary involvement are the most common presentations of COVID-19, the disease can also affect other organs, including the gastrointestinal tract (8, 9). Presentation of gastrointestinal symptoms ranges from 3% to 40% and may even be among the first presentations of the disease (9). Based on the previous studies, liver damage is among the complications of the COVID-19, so that increased level of liver enzymes occurs in 40% to 50% of patients.
2. Objectives
In the present study, we attempted to examine the association between the possible liver damage diagnosed based on elevated liver enzymes and the mortality of COVID-19 patients, as well as the frequency of these lesions in Iranian COVID-19 hospitalized patients.
3. Methods
In the present study, 560 hospitalized patients with a confirmed diagnosis of COVID-19 referred to Firoozgar Hospital, a referral teaching hospital in Tehran, between March 1 and 30 April were included. Medical records of patients were used to collect the required data. Confirmed cases were defined based on a positive test of high-throughput sequencing or real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of pharyngeal or nasal specimens (10). A team of expert clinicians and researchers collaborated to extract the data. The collected data were fed into a computerized database, and then the data cleaning and re-checking processes were performed. The data collection was performed while the patients were hospitalized; as a result, the research team could manage the possible missing data.
The patients’ death was considered as the outcome. In addition to liver enzymes [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)], demographic data (sex and age), clinical data (temperature, systolic blood pressure, the type of treatment in hospital stay duration, respiratory rate, pulse rate, diabetes mellitus status, coronary artery status, and immune system status), COPD/Asthma status, and some other laboratory data [alkaline phosphatase, bilirubin, white blood count (WBC) and platelet count] were collected. Liver enzymes were measured at the time of admission and on the third day. ALT and AST levels ≥ 40 were considered abnormal (10).
3.1. Statistical Analysis
Descriptive statistics were presented as mean (± SD), median (IQR), and proportions (95% CI). After evaluating the normality of data, the two groups were compared using the independent sample t-test (for normally distributed data) and Mann-Whitney test (for data that were not normally distributed). Also, the related proportions were compared between two groups using classical tests of the hypothesis (two-group proportion test) in STATA version 12 (STATA Corp., Texas, USA). Also, multiple logistic regression was used to assess the association between abnormal levels of liver enzymes (on the admission day, the third day of admission, and discharge day) and death. In this analysis, age, SBP, diabetes mellitus, immunodeficiency status, coronary artery disease status, COPD/asthma status, respiratory rate, pulse rate, temperature, and the type of treatment during hospitalization were considered as potential mediators. Multicollinearity was evaluated, which was found to be sound. The all variance inflation factors (VIF) had a range of 1.014 to 2.490. Statistical significance was considered when P-value < 0.05. Statistical analyses were performed using STATA version 12 (STATA Corp., Texas, USA) and SPSS version 21 (Inc., Chicago statistical software).
4. Results
Of 560 patients with COVID-19, 320 (58.4%) were male. Based on our results, 29.1% (95% CI = 25.3% - 32.9%) of participants had a high level (≥ 40 U/Liter) of ALT, and 45.1% (95% CI = 40.9% - 49.3%) had a high level of AST (≥ 40 U/Liter). Death occurred in 83 patients (14.8%). The mean duration of hospitalization was 8.10 ± 5.29. The basic characteristics of the participants are provided in Table 1. Mean age was 59.17 ± 15.93 and median of (Interquartile range = IQR) AST, ALT, and Alkaline phosphatase values were 36.00 (28.00), 29.00, and 165.00 (84.5), respectively. Other data are also provided in Table 1. Based on the results, there was no significant association between sex and death. While 57.7% (275/477) of patients who survived were male, the percentage of the patients who died (62.7%, 52/83) was also insignificantly higher in men (P-value = 0.394).
| Variable | Values |
|---|---|
| Age, mean ± SD, y | 59.17 ± 15.93 |
| SBP, mean ± SD, mmHg | 118.34 ± 13.04 |
| Respiratory rate, median (IQR) | 18.00 (4.00) |
| Pulse rate, median (IQR) | 80.00 (11.50) |
| Temperature, mean ± SD, °C | 37.02 ± 3.13 |
| AST, median (IQR), U/Liter | 36.00 (28.00) |
| ALT, median (IQR), U/Liter | 29.00 (23.00) |
| Alkaline phosphatase, median (IQR), U/Liter | 165.00 (84.5) |
| Bilirubin (total), median (IQR), mg/dL | 0.7 (0.4) |
| Bilirubin (direct), median (IQR), mg/dL | 0.3 (0.2) |
| WBC, median (IQR), mL | 6100 (3700.00) |
| Platelet, median (IQR), mL | 185000.00 (84500.00) |
| People with DM-based on % (95% CI) | 26.1 (22.4 - 29.7) |
| People with CAD-based on % (95% CI) | 18.0 (14.8 - 21.2) |
| Immunodeficient people-based on % (95% CI) | 6.1 (4.1 - 8.1) |
| Smoker people-based on % | 10.8 (4.0 - 17.6) |
| People with COPD or asthma-based on % | 13.2 (5.8 - 20.7) |
| Steroid use-based on % (95% CI) | 21.5 (17.9 - 25.2) |
| IV IG use-based on % (95% CI) | 7.3 (5.0 - 9.6) |
| N-acetyl cysteine use-based on % (95% CI) | 16.1 (12.8 - 19.3) |
| Kaletra use-based on % (95% CI)a | 49.8 (45.4 - 54.2) |
| Hydroxychlorquine use-based on % (95% CI) | 86.0 (83.0 - 89.0) |
| Ribavirin use-based on % (95% CI) | 23.3 (19.7 - 26.9) |
| Sofosbuvir use-based on % (95% CI) | 50.1 (45.8 - 54.4) |
Abbreviations: ALT, denotes alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; IQR, interquartile range; IV IG, intravenous immunoglobulin; SBP, systolic blood pressure; SD, standard deviation.
aLopinavir/ritonavir.
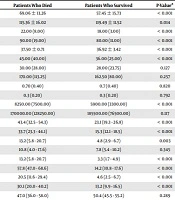
The basic characteristics and the potential predictors of death in patients who survived and died are provided in Table 2. Age (P-value < 0.001), respiratory rate (P-value < 0.001), systolic blood pressure (P-value = 0.014), pulse rate (P-value < 0.001), temperature (P-value < 0.001), AST (P-value < 0.001), WBC count (P-value < 0.001), immunodeficiency status (P-value = 0.003), diabetes mellitus (P-value < 0.001), and coronary artery disease (P-value < 0.001), COPD/asthma (P-value < 0.001) had a significant association with death. Moreover, a higher proportion of people who died were treated with drugs such as steroids (P-value < 0.001), IV IG (P-value < 0.001), and N-acetyl cysteine (P-value < 0.001), in comparison to those who survived.
| Variable | Patients Who Died | Patients Who Survived | P-Valuea |
|---|---|---|---|
| Age, mean ± SD, y | 69.06 ± 13.26 | 57.45 ± 15.73 | < 0.001 |
| SBP, mean ± SD, mmHg | 115.36 ± 16.02 | 119.49 ± 11.52 | 0.014 |
| Respiratory rate, median (IQR), no | 22.00 (8.00) | 18.00 (3.00) | < 0.001 |
| Pulse rate, median (IQR), no | 90.00 (19.00) | 80.00 (11.00) | < 0.001 |
| Temperature, mean ± SD, °C = | 37.50 ± 0.71 | 36.92 ± 3.42 | < 0.001 |
| AST, median (IQR), U/Liter | 45.00 (40.00) | 36.00 (25.00) | < 0.001 |
| ALT, median (IQR), U/Liter | 30.00 (28.00) | 28.00 (23.75) | 0.127 |
| Alkaline phosphatase, median (IQR), U/Liter | 170.00 (113.25) | 162.50 (80.00) | 0.257 |
| Bilirubin (total), median (IQR), mg/dL | 0.70 (0.40) | 0.7 (0.40) | 0.820 |
| Bilirubin (direct), median (IQR), mg/dL | 0.3 (0.20) | 0.3 (0.20) | 0.792 |
| WBC, median (IQR), per mL | 8250.00 (7500.00) | 5900.00 (3300.00) | < 0.001 |
| Platelet, median (IQR), per mL | 170000.00 (128250.00) | 185500.00 (76500.00) | 0.117 |
| People with DM-based on % (95% CI) | 43.4 (32.5 - 54.3) | 23.1 (19.3 - 26.8) | < 0.001 |
| People with CAD-based on % (95% CI) | 33.7 (23.3 - 44.1) | 15.3 (12.1 - 18.5) | < 0.001 |
| Immunodeficient people-based on % (95% CI) | 13.2 (5.8 - 20.7) | 4.8 (2.9 - 6.7) | 0.003 |
| Smoker people- based on % | 10.8 (4.0 - 17.6) | 7.8 (5.4 - 10.2) | 0.345 |
| People with COPD or asthma-based on % | 13.2 (5.8 - 20.7) | 3.3 (1.7 - 4.9) | < 0.001 |
| Steroid use-based on % (95% CI) | 57.8 (47.0 - 68.6) | 14.2 (10.8 - 17.6) | < 0.001 |
| IV IG use-based on % (95% CI) | 20.5 (11.6 - 29.4) | 4.6 (2.5 - 6.7) | < 0.001 |
| N-acetyl cysteine use-based on % (95% CI) | 30.1 (20.0 - 40.2) | 13.2 (9.9 - 16.5) | < 0.001 |
| Kaletra use-based on % % (95% CI) | 47.0 (36.0 - 58.0) | 50.4 (45.5 - 55.2) | 0.289 |
| Hydroxychlorquine use-based on % (95% CI) | 85.5 (77.8 - 93.2) | 86.1 (82.7 - 89.4) | 0.876 |
| Ribavirin use-based on % (%95CI) | 28.9 (18.9 - 38.9) | 22.2 (18.3 - 26.1) | 0.185 |
| Sofosbuvir use-based on % (95% CI) | 45.8 (34.8 - 56.7) | 50.9 (46.3 - 55.5) | 0.393 |
Abbreviations: ALT denotes, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; IQR, interquartile range; IV IG, Intravenous immunoglobulin; SBP, systolic blood pressure; SD, standard deviation.
aMean age of the two groups was compared by independent t-test. Medians were compared by the Mann-Whitney test. The proportions were compared using the two-group proportion test.
Based on our results, the frequency (based on percentage) of a high level of AST (≥ 40 U/Liter) was significantly higher in patients who died [67.3% (95% CI = 54.5% - 80.1%] than those who survived [44.9% (95% CI = 39.7% - 50.0%)] (P-value < 0.001). There was no significant difference concerning the ALT level between expired [29.1% (95% CI = 16.7% - 41.5%)] and survived patients [30.7% (95% CI = 25.9% - 35.5%] (P-value = 0.791) (Table 3).
| Variable | Status | Percent (95% CI) | P-Value | |
|---|---|---|---|---|
| Patients Who Died | Patients Who Survived | |||
| AST (at the first time of hospitalization) | < 40 | 32.7 (19.9 - 45.5) | 55.1 (50.0 - 60.2) | < 0.001 |
| ≥ 40 | 67.3 (54.5 - 80.1) | 44.9 (39.7 - 50.0) | ||
| ALT (at the first time of hospitalization) | < 40 | 70.9 (58.5 - 83.3) | 69.2 (64.5 - 74.0) | 0.791 |
| ≥ 40 | 29.1 (16.7 - 41.5) | 30.7 (25.9 - 35.5) | ||
| AST (at the third day) | < 40 | 35.1 (22.3 - 47.9 | 56.7 (51.6 - 61.8) | 0.025 |
| ≥ 40 | 64.9 (52.1 - 77.7 | 43.3 (38.2 - 48.4) | ||
| ALT (at the third day) | < 40 | 49.1 (35.7 - 62.5) | 48.5 (43.3 - 53.7) | 0.929 |
| ≥ 40 | 50.9 (37.5 - 64.3) | 51.5 (46.3 - 56.7) | ||
Abbreviations: ALT, denotes alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval.
To remove the effects of potential mediators of death and AST status, we conducted multiple logistic regression analyses in which age, SBP, diabetes mellitus, immunodeficiency status, coronary artery disease status, asthma and COPD status, respiratory rate, pulse rate, temperature, and drug use were considered potential mediators. AST on admission showed an independent association with death in the multiple logistic regression analysis (Wald = 4.429, odd ratio [OR] (95% CI) = 1.014 (1.008 - 1.020), P-value = 0.035). A significant association was also detected between AST at the third day of admission and death (Wald = 10.78, odd ratio [OR] (95% CI) = 1.046 (1.018 - 1.074), P-value = 0.001). No significant association was detected between AST change (from the first day of hospitalization to the third day) and death. The results of multiple logistic regression analyses are reported in Table 4 (the results of multiple regression analyses are described Tables S1, S2, and S3 in Supplementary File).
| Variables | Wald | Odd Ratio (95%) | P-Value |
|---|---|---|---|
| ALT (at the first time of hospitalization) | 3.984 | 0.993 (0.985 - 1.000) | 0.046 |
| AST (at the first time of hospitalization) | 4.429 | 1.014 (1.008 - 1.020) | 0.035 |
| ALT (on the third day) | 8.66 | 0.973 (0.955 - 0.991) | 0.003 |
| AST (on the third day) | 10.78 | 1.046 (1.018 - 1.074) | 0.001 |
| ALT change (from at the first time of hospitalization to third day) | 2.537 | 1.006 (0.999 - 1.012) | 0.111 |
| AST change (from at the first time of hospitalization to third day) | 1.264 | 1.007 (0.995 - 1.020) | 0.261 |
Abbreviations: ALT, denotes alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval.
5. Discussion
This study demonstrated a significant association between AST and the death of COVID-19 hospitalized patients. This association was significant when we removed the effects of potential mediators, including age, underlying diseases, such as coronary artery disease, diabetes mellitus, and systolic blood pressure, COPD/asthma, immune function status history, and the type of treatment during the hospitalization. Based on the results, with a single U/Liter increase in AST level, the chance of death increases by 1.4% [(odd ratio (95% CI) = 1.014 (1.008 - 1.020) and P-value = 0.035 for AST in multiple logistic regression with death as outcome]. As noted before, this association was obtained by removing the effects of other potential mediators. However, in the present study, ALT and some other liver markers, such as bilirubin level and alkaline phosphatase, did not show a significant association with death.
Overall, patients with more severe forms of COVID-19 appear to be more likely to have liver disorders. Huang et al. (11) reported an increased level of enzyme in 62% of patients (8 out of 13) with COVID-19 who were hospitalized at the intensive care unit (ICU), compared to only 25% (7 out of 25) of those who did not require ICU hospitalization. Guan et al. (10) reported an increased level of liver enzyme in patients with more severe forms of COVID-19. Also, another study reported that people in the subclinical phase had a lower level of AST compared to those in the clinical phase (12). Cai et al. (13) found that patients with COVID-19, who had abnormal levels of liver enzymes, were more likely to develop more severe forms of the disease. In a systematic review, Parohan et al. (14) showed that higher serum levels of AST, ALT, and total Bilirubin, as well as lower serum levels of Albumin, were associated with a significant increase in the severity of COVID-19 infection.
However, in the present study, as mentioned above, only AST showed an independent association with death. The observed difference in the results of various studies can be attributed to the difference in investigated outcomes. The difference in interesting outcomes can be the main cause of the observed difference in the findings of previous studies. On the other hand, overall, in patients with COVID-19, the AST level is more likely to be elevated compared to ALT (15). This may be because AST, in addition to liver involvement, also increases when other organs are involved, as well (15). As a result, in addition to damaging the liver, an increased level of AST may indicate damage to other organs, making the patient experience even worse consequences when this enzyme rises. Thus, a worse prognosis can be expected in people with COVID-19 when AST is elevated in comparison to cases with elevated ALT. Our study also showed that 29.1% of participants had a high level of ALT (≥ 40 IU). Also, we found that 45.1% of our study’s population had a high level of AST (≥ 40 IU). Previous studies showed that about 60% of people with SARS had liver damage, and SARS coronavirus could be found in hepatocytes, too (16).
In vitro studies have also confirmed that SARS coronavirus causes liver lesions (17). Moreover, abnormal AST and ALT levels are reported as 22.2% and 21.3% in people with COVID-19 by Guan et al, respectively (10). Based on the results of the present study, 67% of the subjects with COVID-19 who died had a higher level of AST. This finding is in line with the findings reported by a previous study, which showed that the presence of liver lesions in people who died due to COVID-19 was 58% to 78% (18). The underlying mechanisms for liver involvement in COVID-19 are not fully understood. However, the high presence of angiotensin 2 receptors in the small intestine and the high blood flow from the small intestine to the liver can provide the basis for the presence of the virus in the liver. On the other hand, there are a lot of liver macrophages that can produce cytokines, which provides a basis for liver damage (19). Iatrogenic hepatic injury can also play a role in causing liver damage in more severe forms of the COVID-19. For instance, positive end-expiratory pressure in mechanical ventilation can cause hepatic congestion by increasing right atrial pressure as well as preventing venous return (20). Drug hepatotoxicity during the treatment period, immune-mediated inflammation, and pneumonia-associated hypoxia might also be other causes of liver damage in severe forms of COVID-19 (21).
Our study showed that liver involvement, defined by high levels of liver enzymes, is a common finding in COVID-19 hospitalized patients. We also found that AST may be a good independent predictor of severe forms of COVID-19 that can even lead to death. As a result, the clinicians should monitor the status of hepatic involvement in people with COVID-19 at the onset of consultation and during treatment. This is especially important for the AST, as it has been shown to be linked to adverse effects, particularly death, and an increase in this enzyme may be a marker of the severity of the disease. However, we couldn’t find any association between death and other evaluated markers of liver involvement, such as bilirubin and alkaline phosphatase. Also, it should be noted that although we found that a high proportion of hospitalized patients had a high level of liver enzymes, a part of this high proportion may be due to the lesions that had previously been developed, which patients were unaware of them. The current study had limitations. Elevated liver enzymes can be a common condition in the general population, and relevant information were not available for many patients. However, as noted, the current study aimed to investigate the association between liver enzymes and in-hospital mortality. This finding can be used by clinicians as a potential criterion for classifying patients based on their risk, particularly regarding the AST. Furthermore, case-control studies are not sufficient for confirming cause and effect associations. Although the hypothetical cause (increase in liver enzymes) occurred before the outcome, the other Hill’s criteria may not be completely addressed by our study.
5.1. Conclusions
This study demonstrated that liver involvement is a common finding in COVID-19 hospitalized patients. Besides, we found a significant and independent association between AST and death.