1. Background
Chronic hepatitis C virus (HCV) infection causes progressive liver damage, which may result in liver cirrhosis and hepatocellular carcinoma (HCC). Worldwide, between 64 and 103 million people are chronically infected with this infection (1), with a HCV antibody prevalence of 0.3% in Germany, which is among the countries with low HCV prevalence (2).
It is well-proved that anti-HCV therapy can diminish the burden of end-stage liver disease and HCV-related extrahepatic manifestations. The recent introduction of direct-acting antivirals (DAAs) has revolutionized the treatment of chronic HCV infection with a high sustained virological response (SVR) and good safety and tolerability profile (3).
Glecaprevir/pibrentasvir (GLE/PIB) is the latest DAA approved by administrative agencies in the United States and Europe. It is a fixed-dose combination of a NS3/4A protease inhibitor (glecaprevir) and a NS5A inhibitor (pibrentasvir) (4). GLE/PIB contains 100 mg/40 mg and is administered orally once a day. The regimen achieved high SVR across all HCV genotypes (GT) and was confirmed to be highly efficacious in difficult-to-treat patient subgroups such as compensated cirrhosis, severe renal impairment, and patients with previous DAA failure (5-8). The tolerability of GLE/PIB is well proven, and adverse events (AEs) are rarely described. The regimen was approved in July 2017 by the European Medicine Agency (EMA) for treating chronic HCV infection with advanced hepatic fibrosis or compensated cirrhosis (9).
Because patients with unfavorable factors (e.g., older age, co-morbidities, or concurrent malignancy) are commonly excluded from clinical trials, the collected data may be an overestimation of reality. Data of real-world cohorts help clinicians to better understand the regimen used for daily clinical practice. Regarding GLE/PIB, real-world data are limited and, to date, published out of the context of registry-based studies (10-12). To evaluate the effectiveness and safety of GLE/PIB in a real-life setting, we retrospectively analyzed data from unselected HCV-positive patients from a German tertiary referral center.
2. Objectives
Hence, in the present study, we presented characteristics and treatment results of an unsponsored, single-center study of a German cohort of patients with chronic HCV infection.
3. Methods
A total of 100 consecutive patients with chronic HCV infection were enrolled into this single-center, retrospective cohort study. All patients presented at the outpatient clinic for infectious diseases and viral hepatitis of the University Medical Center Hamburg-Eppendorf, Germany, from October 2017 to September 2019. The center is a tertiary care referral center for viral hepatitis in Germany. Patients are referred by general practitioners or specialized gastroenterologists and hepatologists of the whole northern part of Germany.
The primary endpoint of the study was defined as a sustained viral response (SVR) 12 weeks after the end of treatment (EoT). Patient charts were analyzed with a focus on demographics, clinical data (stage of liver fibrosis; pretreatment; therapy duration; response to treatment; side effects and co-infections with HIV and/or hepatitis B virus infection) as well as laboratory measurements such as HCV GT and HCV RNA at different time points. Liver cirrhosis was diagnosed non-invasively using transient elastography (TE) with a liver stiffness cut-off of 12.5 kPa as described in the literature (13). Here, we presented the characteristics and treatment results of an unsponsored, single-center study of a German cohort of patients with chronic HCV infection.
4. Results
As mentioned before, 100 unselected patients were scheduled to receive GLE/PIB. However, one patient did not receive the treatment due to an early stage of pregnancy (1/100; 1%). Hence, 99 (99/100; 99%) HCV infected patients consecutively started therapy with GLE/PIB (100 mg/40 mg).
The median age of patients was 48 years at the start of therapy. Also, 37% of patients were female (n = 37). The mean viral load before starting the treatment was 2,320,000 U/L (ranged from 1,740 to 26,900,000 U/L).
For most of the participants (n = 60; 60%), the transmission mode of HCV was unknown, with intravenous drug abuse (IVDA) as the greatest risk factor (24/100; 24%) (see Table 1).
| Patient Characteristics | Values |
|---|---|
| Patients, N | 100 |
| Age | 48 (18/79) |
| Sex, male/female (%) | 63/37 (63/37) |
| Genotype | |
| 1a | 28 (28) |
| 1b | 26 (26) |
| 1, not further specified | 1 (1) |
| 2 | 8 (8) |
| 3 | 25 (25) |
| 4 | 8 (8) |
| 5 | 1 (1) |
| 6 | 1 (1) |
| Unknown | 2 (2) |
| Transmission | |
| IVDA | 24 (24) |
| Transfusion | 9 (9) |
| Sex | 4 (4) |
| Tatoo | 3 (3) |
| Unknown | 60 (60) |
| Liverbiopsy | 0 (0) |
| Liver stiffness, kPa | 89 (89) |
| < 6 | 44 (49) |
| > 6 | 45 (50) |
| > 12,5 | 3/46 (6) |
| No liver elastography done | 11 (11) |
| HCV RNA (U/L) pre-treatment | 2 320 000 (1 740/26 900 000) |
| Alanine transaminase (U/L) pre-treatment | 70 (14 - 3277) |
| Alanine transaminase (U/L) post-treatment | 22 (10 - 192) |
| Pre-treatment with DAA | 0 (0) |
| Pre-treatment with IFN | 10 (10) |
| Treatment duration, wk | |
| 4 | 1 (1) |
| 8 | 96 (97) |
| 12 | 1 (1) |
| 16 | 1 (1) |
| Treatment discontinuation | 0 (0) |
| Lost to follow-up | 22 (22) |
| Clinical visits and follow-ups (from start of treatment to SVR12) | 4/4 (1 - 7) |
| Co-infections | |
| Previous HBV infection (HBsAG and VL negative) | 22 (22) |
| Chronic HBV infection (HBcAG and HBsAG positive) | 4 (4) |
| Chronic HIV-1 infection | 4 (4) |
| Transplantation | |
| Liver and kidney transplantation | 1 (1) |
| Kidney transplantation | 1 (1) |
| TIPS | 0 (0) |
Abbreviations: DAA, direct antiviral agents; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN, interferons; IVDA, intravenous drug abuse; kPa, kilopascals; RNA, ribonucleic acid; TIPS, transjugular intrahepatic portosystemic shunt.
aValues are expressed as No. (%) or median (min/max) or mean/median (range).
GT 1 was the most prevalent GT in our cohort (1a: 28/100; 28%, 1b: 26/100; 26%; GT 1 not further specified: 1/100; 1%), followed by GT 3 (25/100; 25%). In only two patients genotyping was not performed and was unknown (see Table 1).
TE was available in 89 patients, where 50% of patients (45/89) had an elevated measurement (6-12,5 kPa), indicating fibrosis of the liver. The number of treated patients with pre-existing cirrhosis, indicated by liver stiffness values of > 12.5 kPa, was low (3/89; 3%). No patient had a history of liver biopsy.
Ten patients were pre-treated with IFN containing regiments (10/100; 10%). While two of them were lost to follow-up (2/10; 20%), eight patients achieved SVR12 (8/10; 80%). None of our patients received previous treatment with DAA.
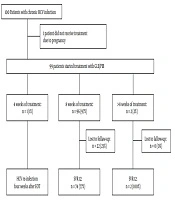
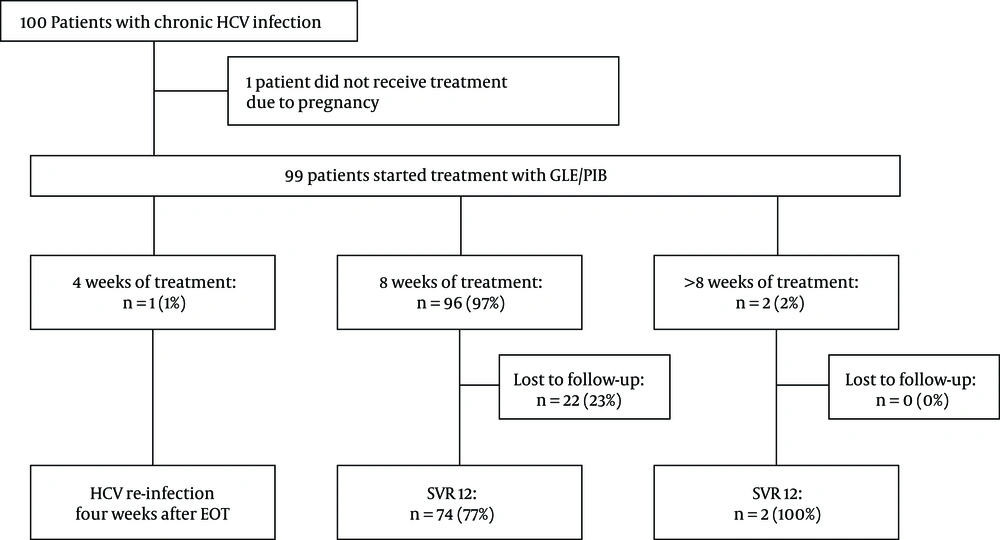
Treatment duration ranged from 4 to 16 weeks. The majority of patients received 8 weeks of treatment (96/99; 97%). Due to poor compliance, one patient discontinued the treatment after 4 weeks (GT 3; liver stiffness 4,8 kPa). One patient received 12 weeks of treatment (GT 1b; liver stiffness 7,9 kPa) due to unknown reasons. Another patient with suspected cirrhosis, hence the treatment duration was extended 16 weeks. The allocation of treatment and patients’ follow-up are summarized in Figure 1.
Allocation of treatment. In total 100 patients were included in our retrospective cohort study, in which 99 of them received treatment (99%). Ninty-six patients (97%) received eight weeks of therapy. A high number of patients (22/96; 23%) were lost to follow up. Only three patients received less or more than 8 weeks of therapy, where one patient experienced HCV re-infection four weeks after the end of treatment and two achieved SVR12 (n = 2; 100%). Abbreviations: HCV, hepatitis C virus; GLE/PIB, glecaprevir/pibrentasvir; SVR 12, sustained virological response rate at week 12; EOT, end of treatment.
All patients who were followed up (76/99; 77%) achieved SVR12 (76/76; 100%) (see Figure 1). Generally, the safety and tolerability of HCV treatment were very good. Only four patients (4/99, 4%) reported treatment-related adverse events, such as nausea and headaches. In patients with advanced fibrosis, no hepatic decompensation was seen during treatment. No treatment discontinuation due to adverse events was observed. However, many patients were lost to follow-up after the end of treatment (22/99; 22%).
Four patients were chronically co-infected with hepatitis B virus (HBV). One HBV co-infected patient showed increased viral load during anti-HCV treatment at week four and developed signs of acute hepatitis (GPT at EoT: 192 U/L). HBV viral load decreased spontaneously, and liver enzymes normalized within 12 weeks after EoT. Twenty-two patients (22/100; 22%) had previous HBV infection (HBcAg positive; HBsAg negative; anti-HBs positive). HBsAg and HBV viral load were closely monitored, but none of them showed signs of reactivation during treatment with GLE/PIB.
Four patients (4/100; 4%) were HIV-1 co-infected and received highly active antiretroviral therapy (HAART) with good treatment adherence and response. One of those was also chronically co-infected with HBV.
A 26-year old patient with GT 3 discontinued treatment with GLE/PIB after four weeks due to poor compliance. The patient’s follow-up visits were irregular. Eight weeks after beginning the treatment, HCV viral load was undetectable, while 16 weeks after EoT, a high HCV viral load (25,200,000 U/L) and signs of acute hepatitis were observed (GPT 84 U/L, GOT 80 U/L). Surprisingly, genotyping revealed HCV re-infection with GT 1b. Re-treatment with sofosbuvir/velpatasvir was initiated for twelve weeks. To date, HCV viral load was undetectable at EoT.
Two patients underwent solid organ transplantation prior to treatment with GLE/PIB. Both patients achieved SVR12 without any adverse event.
Ideally, patients should be followed-up at least seven times during and after the treatment at our outpatient clinic, every two to four weeks (i.e. initially, two weeks after starting the treatment, week four, week eight, SVR four, SVR eight, and SVR twelve). In real-life, most patients miss at least one appointment (95/99, 96%). The median of follow-up visits was four, with an average of four visits. While only five patients (5/99; 5%) were seen as scheduled for seven follow-up visits, seven (7/99; 7%) only had the initial visit (prior to starting the treatment).
5. Discussion
In real life, there are significant differences between the general population and patient groups. Besides, patients’ compliance to treatment may be lower than those in clinical studies. Therefore, assessing therapeutic success in the real world is of crucial importance.
In our retrospective data analysis, we found that GLE/PIB was effective and well-tolerated in 99 unselected patients with chronic HCV in real-world settings. In patients with regular follow-up visits, the over-all SVR12 was achieved in 100% (76/76). In a comprehensive meta-analysis about real-world effectiveness and safety of GLE/PIB, Lampertico et al. (14) evaluated 18 cohorts with a total of 12 531 patients. Ther reported a SVR12 of > 95% across all subgroups. Adverse events were reported as low in 17,7% of patients. Consistent with our data, the authors concluded that GLE/PIB is a well-tolerated and highly effective therapeutic option for pan-genotypic HCV treatment.
The median age of our cohort was 48 years, the youngest and oldest patients were 18 to 79 years old, respectively. Published approval studies included comparable age groups (15-17). More males than females started treatment with GLE/PIB. No sex-specific difference was observed concerning virological response or adverse events. This is also true for data from clinical trials (15-17).
In accordance with European epidemiological data, in the present study, the most prevalent GT was GT 1, followed by GT 3 (18). The greatest risk factor associated with HCV transmission was IVDA in 24 patients (24/100; 24%), which is in line with recently published data where IVDA is reported as the leading mode of acute HCV infection transmission (19).
GLE/PIB is reported to be highly effective the in re-treatment of patients, particularly in patients with failed interferon-based regimens (16, 17, 20). While 10% of the presented patients received treatment with IFN containing regimens prior to therapy with GLE/PIB, 90% of patients were treatment-naive. Accordingly, none of the patients received previous treatment with DAA. SVR12 was achieved in eight of the pre-treated patients (8/10; 80%), while two pre-treated patients were lost to follow-up. Owing to the small number of re-treated patients in our cohort, GLE/PIB seems to be effective and safe for retreatment of HCV, but further evidence are required.
Although GLE/PIB is approved for treating patients with advanced fibrosis and compensated cirrhosis, in our cohort, only three patients (3/100; 3%) presented signs of advanced fibrosis. This suggests that, in a real-life setting, GLE/PIB is the treatment of choice for patients with no or low-grade fibrosis, while patients with advanced fibrosis or cirrhosis were probably treated with other fixed-dose combination regimens, such as sofosbuvir/velpatasvir or elbasvir/grazoprevir. One advantage of treatment with GLE/PIB might be the short treatment duration (eight weeks) in patients without cirrhosis compared to other DAA regimens. Until recently, in patients with compensated cirrhosis - treatment-naive or treatment-experienced-therapy with GLE/PIB was extended up to 12 and 16 weeks, respectively (16, 21). Lately, Brown et al. showed that GLE/PIB led to similarly high SVR12 in a short 8-week regimen compared to the formerly approved 12-week regimen in treatment-naive patients with compensated cirrhosis, which is now also approved in Europe (16). Real-world data of patients with advanced fibrosis and/or compensated cirrhosis treated with a short 8-week GLE/PIB regimen are still pending, and further evaluations are required.
Patient adherence is a key factor in clinical trials aiming to achieve high efficacy. In real-world settings, adherence is oftentimes worse, as also shown in our study. Patient-related factors that may negatively influence adherence include, among other things, drug abuse (22, 23), which was the most common risk factor for HCV transmission in the present study (24/100; 24%).
In this cohort study, the lost to follow-up rate for SVR-12 was high, mostly after end of treatment (22/99; 22%). These patients were associated with a greater risk factor of IVDA (10/22; 45%) compared to those who achieved SVR12 (14/76; 18%). Our data indicate that SVR12 depends more on patient compliance than on drug efficacy. Thus, in a cohort of difficult-to-treat patients, finding novel techniques to increase patients’ compliance is important. The short treatment duration of eight weeks seems to be an advantage in these difficult-to-treat patients and also may improve patients’ adherence (24). Although we comprehensively informed our patients about the importance of treatment adherence and follow-up visits during the cost-intensive treatment, most of them (95/99; 96%) missed at least one follow-up visit, which reflects poorer adherence in our cohort. One patient even discontinued treatment after 4 weeks due to non-compliance. Twelve weeks after EoT, the patient presented with a HCV re-infection that was re-treated with a 12-week sofosbuvir/velpatasvir regime.
The safety and tolerability of GLE/PIB are well-established. The most commonly described mild side effects are fatigue, headache, and nausea, by up to 70% in clinical trials (16, 21). Overall, side effects were rarely reported in our cohort. Only four patients presented mild side effects like nausea and headache (4/99; 4%). These differences can be attributed to underreporting or under documentation by treating physicians. Apart from chronic HCV infection, patients of our cohort were healthy individuals with a small proportion of advanced liver fibrosis, which may also contribute to the fact that few adverse events were observed.
During DAA treatment, HBV co-infected patients are at risk of reactivated infection or experiencing a flare of HBV (25). Cases of acute liver failure and even fatal cases are reported (26). HBsAg-positive individuals are excluded from clinical trials and HBV reactivation is only reported after DAAs entered into the clinical practice. In our cohort, four patients (4/100; 4%) were chronically co-infected with HBV infection (HBsAG positive, HBV DNA positive). A high number of patients (22/100; 22%) were previously co-infected with HBV, who were defined as HBcAg positive, HBsAg negative, and anti-HBs positive.
Ma and Feld (25) recommends closely monitor HBV DNA and even starting pre-emptive anti-HBV treatment until SVR12 in HBsAg-positive and positive HBV DNA positive patients at baseline. One patient with chronic HBV co-infection showed an increase of HBV viral load and laboratory signs of acute hepatitis during the course of treatment. However, at SVR12, HBV DNA decreased, and liver enzymes normalized without any specific treatment.
Four patients (4/100; 4%) were HIV-1 co-infected. All of them completed eight weeks of treatment with GLE/PIB without any reported adverse event. While three patients achieved SVR12 (3/4; 75%), one was lost to follow-up at SVR8 (HCV viral load undetectable). These high response rates are in line with the EXPEDITION-2 study (2018), where GLE/PIB was shown to be a highly efficacious and well-tolerated treatment for HCV/HIV-1 co-infected individuals (27).
Only two patients had a history of solid organ transplantation before receiving GLE/PIB treatment. In 2018, Reau et al. (28) published a retrospective analysis of 100 patients who underwent a liver or kidney transplant and received GLE/PIB for 12 weeks. They concluded that GLE/PIB was well-tolerated and effective in this special cohort.
The current study had limitations, including a small sample size and using a non-standardized method to collect clinical data. Patient’s loss to follow-up is not uncommon in every-day clinical practice and can give insights into the compliance of a difficult-to-treat patient cohort.
In conclusion, in the present study, therapy with GLE/PIB was conducted in generally healthy individuals with a low percentage of pre-existing liver fibrosis in this real-life setting. Our study confirms excellent effectiveness and safety of treatment with GLE/PIB for 8 weeks. Additionally, our study shows and confirm that besides efficiency, patient adherence is essential to achieve SVR12.